Abstract
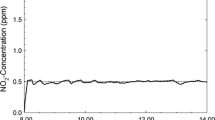
Exhaled endogenous nitric oxide (NO) holds promise as a potential biomarker of pulmonary inflammation. Previous experimental and theoretical work has concluded that the alveolar concentration approaches a constant steady state value at end exhalation due to both a constant maximum flux or release of NO (Jmax,alv) and a constant diffusing capacity (DNO,alv) in the alveolar region. We have recently demonstrated that DNO,alv is not constant, but increases with alveolar volume (VA) given by the following average relationship: DNO,alv=48*V 2/3A ml/min/mmHg (where VA is expressed in liters, STPD). We investigated the potential impact of a variable DNO,alv on exhaled concentration by incorporating the volume dependence into the currently accepted two-compartment model for NO exchange dynamics. Our results suggest that the mechanism underlying the plateau in exhaled concentration is a constant ratio Jmax,alv/DNO,alv.This constant ratio requires a volume dependence of Jmax,alv similar to DNO,alv, and is likely due to a decreasing alveolar surface area during exhalation. © 2001 Biomedical Engineering Society.
PAC01: 8719Uv, 8710+e, 8715Vv, 8239Rt
Similar content being viewed by others
References
Alving, K., E. Weitzberg, and J. M. Lundberg. Increased amount of nitric oxide in exhaled air of asthmatics. Eur. Respir. J.6:1368–1370, 1993.
Bannenberg, G. L., and L. E. Gustafsson. Stretch-induced stimulation of lower airway nitric oxide formation in the guinea-pig: Inhibition by gadolinium chloride. Pharm. Toxicol.81:13–18, 1997.
Davidson, M. R., and J. M. Fitzgerald. Transport of O2 along a model pathway through the respiratory region of the lung. Bull. Math. Biol.36:275–303, 1974.
DuBois, A. B., P. M. Kelley, J. S. Douglas, and V. Mohsenin. Nitric oxide production and absorption in trachea, bronchi, bronchioles, and respiratory bronchioles of humans. J. Appl. Physiol.86:159–167, 1999.
Fukuto, J. M. Chemistry of nitric oxide: Biologically relevant aspects. In: Nitric Oxide: Biochemistry, Molecular Biology, and Therapeutic Implications, edited by Ignarro and Murad. San Diego: Academic, 1995, pp. 1–15.
Gaston, B., J. M. Drazen, J. Loscalzo, and J. S. Stamler. The biology of nitrogen oxides in the airways. Am. J. Respir. Crit. Care Med. 149:538–551, 1994.
Hung, J. F., K. Fang, R. Malik, A. Snyder, N. Malhotra, T. A. Platts-Mills, and B. Gaston. Endogenous airway acidification. Implications for asthma pathophysiology. Am. J. Respir. Crit. Care Med.161:694–699, 2000.
Hyde, R. W., E. J. Geigel, A. J. Olszowka, J. A. Krasney, R. E. Forster, M. J. Utell, and M. W. Frampton. Determination of production of nitric oxide by lower airways: Theory. J. Appl. Physiol.82:1290–1296, 1997.
Kharitonov, S., K. Alving, and P. J. Barnes. Exhaled and nasal nitric oxide measurements: Recommendations. The European Respiratory Society Task Force. Eur. Respir. J.10:1683–1693, 1997.
Moilanen, E., and H. Vapaatalo. Nitric oxide in inflammation and immune response. Ann. Med.27:359–367, 1995.
Paiva, M., and L. A. Engel. The anatomical basis for the sloping N2 plateau. Respir. Physiol.44:325–337, 1981.
Pietropaoli, A. P., I. B. Perillo, A. Torres, P. T. Perkins, L. M. Frasier, M. J. Utell, M. W. Frampton, and R. W. Hyde. Simultaneous measurement of nitric oxide production by conducting and alveolar airways of humans. J. Appl. Physiol.87:1532–1542, 1999.
Scheid, P., M. P. Hlastala, and J. Piiper. Inert gas elimination from lungs with stratified inhomogeneity: Theory. Respir. Physiol.44:299–309, 1981.
Silkoff, P. E., P. A. McClean, A. S. Slutsky, H. G. Furlott, E. Hoffstein, S. Wakita, K. R. Chapman, J. P. Szalai, and N. Zamel. Marked flow-dependence of exhaled nitric oxide using a new technique to exclude nasal nitric oxide. Am. J. Respir. Crit. Care Med.155:260–267, 1997.
Silkoff, P. E., J. T. Sylvester, N. Zamel, and S. Permutt. Airway nitric oxide diffusion in asthma. Role in pulmonary function and bronchial responsiveness. Am. J. Respir. Crit. Care Med.161:1218–1228, 2000.
Six, D. P., W. R. de Vries, and S. C. Luijendijk. Sloping alveolar plateaus of He and SF6 measured in excised cat lungs ventilated at constant volume by pressure changes. Respir. Physiol.83:277–293, 1991.
Slutsky, A. S., and J. M. Drazen. Recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children 1999. Am. J. Respir. Crit. Care Med.160:2104–2117, 1999.
Staub, N. C., Respiration. Annu. Rev. Physiol.31:173–202, 1969.
Stewart, T. E., F. Valenza, S. P. Reveiro, A. D. Wener, G. Volgyesi, J. Brendan, M. Mullen, and A. S. Slutksy. Increased nitric oxide in exhaled gas as an early marker of lung inflammation in a model of sepsis. Am. J. Respir. Crit. Care Med.151:713–718, 1995.
Tsoukias, N. M., D. Dabdub, A. F. Wilson, and S. C. George. Effect of alveolar volume and sequential filling on the diffusing capacity of the lungs. I. Theory. Respir. Physiol.120:231–250, 2000.
Tsoukias, N. M., and S. C. George. A two-compartment model of pulmonary nitric oxide exchange dynamics. J. Appl. Physiol.85:653–666, 1998.
Tsoukias, N. M., Z. Tannous, A. F. Wilson, and S. C. George. Single-exhalation profiles of NO and CO2 in humans: Effect of dynamically changing flow rate. J. Appl. Physiol.85:642–652, 1998.
Tsoukias, N. M., A. F. Wilson, and S. C. George. Effect of alveolar volume and sequential filling on the diffusing capacity of the lungs. II. Experiment. Respir. Physiol.120:251–271, 2000.
Weibel, E. R., P. Untersee, J. Gil, and M. Zulauf. Morphometric estimation of pulmonary diffusion capacity. VI. Effect of varying positive pressure inflation of air spaces. Respir. Physiol.18:285–308, 1973.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tsoukias, N.M., George, S.C. Impact of Volume-Dependent Alveolar Diffusing Capacity on Exhaled Nitric Oxide Concentration. Annals of Biomedical Engineering 29, 731–739 (2001). https://doi.org/10.1114/1.1397786
Issue Date:
DOI: https://doi.org/10.1114/1.1397786