Abstract
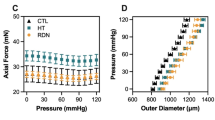
Early stage changes in hypertensive arteries have a significant effect on the long-term adaptation of the arteries. Compared to the long-term adaptation, little is known about the early dimensional and functional changes in hypertensive arteries in the first few days of hypertension. To study the early stage changes in hypertensive arteries, porcine common carotid arteries were cultured for seven days in a simplified ex vivo artery organ culture system with pulsatile flow under hypertensive (200±30 mm Hg) or normotensive (100±20 mm Hg) pressure conditions while maintaining a physiological mean wall shear stress of 15 dyn/cm2.Vessel viability was demonstrated by contractile diameter responses to norepinephrine (NE), carbachol (CCh), and sodium nitroprusside (SNP) as well as staining for mitochondrial activity and cell apoptosis/necrosis. The results show that arteries demonstrated strong contractile responses to NE, CCh, and SNP, basal tone, and viable mitochondria in the organ culture system for seven days. Hypertensive arteries demonstrated a stronger contractile response than normotensive arteries (p < 0.05). Diameter enlargement was observed in hypertensive arteries as compared to arteries cultured under normotensive conditions. In conclusion, the pulsatile culture system can maintain arteries viable with active vasomotion tone for up to seven days. Hypertensive pressure causes arterial adaptation by significantly increasing arterial diameter and contractile response within the first seven days. © 2001 Biomedical Engineering Society.
PAC01: 8719Uv, 8719Rr, 8780Rb
Similar content being viewed by others
References
Bardy, N., G. J. Karillon, R. Merval, J.-L. Samuel, and A. Tedgui. Differential effects of pressure and flow on DNA and protein synthesis and on fibronectin expression by arteries in a novel organ culture system. Circ. Res.77:684–694, 1995.
Bardy, N., R. Merval, J. Benessiano, J.-L. Samuel, and A. Tedgui. Pressure and angiotensin II synergistically induce aortic fibronectin expression in organ culture model of rabbit aorta. Evidence for a pressure-induced tissue renin-angiotensin system. Circ. Res.79:70–78, 1996.
Birukov, K. G., S. Lehoux, A. A. Birukova, R. Merval, V. A. Tkachuk, and A. Tedgui. Increased pressure induces sustained protein kinase C-independent herbimycin A-sensitive activation of extracellular signal-related kinase 1/2 in the rabbit aorta in organ culture. Circ. Res.81:895–903, 1997.
Boutouyrie, P., S. Laurent, A. Benetos, X. J. Girerd, A. P. G. Hoeks, and M. E. Safar. Incompressibility of the human arterial wall: An in vitro ultrasound study. J. Hypertens.10:S87-S91, 1992.
Brant, A. M., M. F. Teodori, R. L. Kormos, and H. S. Borovetz. Effect of variations in pressure and flow on the geometry of isolated canine carotid arteries. J. Biomech.20:831–838, 1987.
Conklin, B. S. Viability of porcine common carotid arteries in a novel organ culture system. Master Thesis, Georgia Institute of Technology, 1997.
Cox, R. H.Comparison of arterial wall mechanics in normotensive and spontaneously hypertensive rats. Am. J. Physiol.237:H159-H167, 1979.
Cox, R. H.Alterations in active and passive mechanics of rat carotid artery with experimental hypertension. Am. J. Physiol.237:H597-H605, 1979.
Davies, P. F.Flow-mediated endothelial mechanotransduction. Physiol. Rev.75:519–560, 1995.
Fung, Y. C. Biomechanics: Stress, Strain and Growth. New York: Springer, 1990, Chaps. 11 and 13.
Furchgott, R. F., and J. Zawadzki. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature (London)288:373–376, 1980.
Han, H. C., B. S. Conklin, G. Hamilton, P. R. Girard, and D. N. Ku. Shear stress modulates the proteinase activity of endothelial cells in porcine carotid artery (Abstract). Ann. Biomed. Eng.25(S1):S19, 1997.
Han, H. C., and D. N. Ku. Smooth muscle function remodels nonuniformly in hypertensive arteries in organ culture. Proceedings of the 3rd World Congress on Biomechanics, Sapporo, Japan (Hokkaido University, 1998), p. 423.
Herman, I. M., A. M. Brant, V. S. Warty, J. Bonaccorso, E. C. Klein, R. L. Kormos, and H. S. Borovetz. Hemodynamics and the vascular endothelial cytoskeleton. J. Cell Biol.105:291–302, 1987.
Holt, C. M., S. E. Francis, S. Rogers, P. A. Gadsdon, T. C. C. Taylor, A. Soyombo, A. C. Newby, and G. D. Angelini. Intimal proliferation in an organ culture of human internal mammary artery. Cardiovasc. Res.26:1189–1194, 1992.
Koo, E. W. Y., and A. I. Gotlieb. Neointimal formation in the porcine aortic organ culture: Cellular dynamics over one month. Lab. Invest.64:743–753, 1991.
Ku, D. N.Blood flow in arteries. Annu. Rev. Fluid Mech.29:399–434, 1997.
Labadie, R. F., J. F. Antaki, J. L. Williams, S. Katyal, J. Ligush, S. C. Watkins, S. M. Pham, and H. S. Borovetz. Pustile perfusion system for ex vivo investigation of biochemical pathways in intact vascular tissue. Am. J. Physiol.270:H760-H768, 1996.
Ligush, J., R. F. Labadie, S. A. Berceli, J. B. Ochoa, and H. S. Borovetz. Evaluation of endothelium-derived nitric-oxide-mediated vasodilatation utilizing ex vivo perfusion of an intact vascular tissue. J. Surg. Res.52:416–421, 1992.
Liu, S. Q., and Y. C. Fung. Relationship between hypertension, hypertrophy, and opening angle of the zero-stress state of arteries following aortic constriction. J. Biomech. Eng.111:325–335, 1989.
Matsumoto, T., and K. Hayashi. Mechanical and dimensional adaptation of rat aorta to hypertension. J. Biomech. Eng.116:278–283, 1994.
Matsumoto, T., E. Okumura, Y. Miura, and M. Sato.Mechanical and dimensional adaptation of rabbit carotid artery cultured in vitro. Adv. Bioeng.33:367–368, 1996.
Mosmann, T.Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assay. J. Immunolog. Meth.65:55–63, 1983.
Palmer R. M. J., A. G. Ferrige, and S. Moncada. Nitro-oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature (London)327:524–526, 1987.
Soyombo, A., G. D. Angelini, A. J. Bryan, and A. C. Newby. Surgical preparation induces injury and promotes smooth muscle cell proliferation in a culture of human saphenous vein. Cardiovasc. Res.27:1961–1967, 1992.
Tanaka, T. T., and Y. C. Fung. Elastic and inelastic properties of the canine aorta and their variation along the aortic tree. J. Biomech.7:357–370, 1974.
White, R., and C. R. Hiley. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesentric artery. Br. J. Pharmacol.122:1573–1584, 1997.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Han, HC., Ku, D.N. Contractile Responses in Arteries Subjected to Hypertensive Pressure in Seven-Day Organ Culture. Annals of Biomedical Engineering 29, 467–475 (2001). https://doi.org/10.1114/1.1376391
Issue Date:
DOI: https://doi.org/10.1114/1.1376391