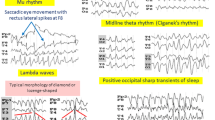
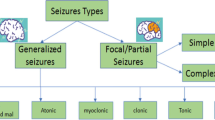
Abstract
Artificial neural networks are widely used for pattern recognition tasks. For spike detection in electroencephalography (EEG), feedforward networks trained by the backpropagation algorithm are preferred by most authors. Opposed to this, we examined the off-line spike detection abilities of a Kohonen feature map (KFM), which is different from feedforward networks in certain aspects. The EEG data for the training set were obtained from patients with intractable partial epilepsies of mesiotemporal (n = 2) or extratemporal (n = 2) origin. For each patient the training set for the KFM included the same patterns of background activity and artifacts as well as the typical individual spike patterns. Three different-sized networks were examined (15 × 15 cells, 25 × 25 cells, and 60 × 60 cells in the Kohonen layer). To investigate the quality of spike detection the results obtained with the KFM were compared with the findings of two board-certified electroencephalographers. Application of a threshold based on the partial invariance of spike recognition against translation of the EEG provided an average sensitivity and selectivity of 80.2% at crossover threshold (71%–86%) depending on the networksize and noise. Multichannel EEG processing in real time will be available soon. In conclusion, pattern-based automated spike detection with a KFM is a promising approach in clinical epileptology and seems to be at least as accurate as other more-established methods of spike detection. © 2000 Biomedical Engineering Society.
PAC00: 8719Nn, 8780-y
Similar content being viewed by others
REFERENCES
Barry, E., N. M. Sussman, M. J. O'Connor, and R. N. Harner. Presurgical electroencephalographic patterns and outcome from anterior temporal lobectomy. Arch. Neurol. 49:21-27, 1992.
Chee, M. W. L., H. H. Morris III, M. A. Antar, P. C. Van Ness, D. S. Dinner, P. Rehm, and V. Salanova. Presurgical evaluation of temporal lobe epilepsy using interictal temporal spikes and positrone emission tomography. Arch. Neurol. 50:45-48, 1993.
Chung, M. Y., T. S. Walczak, D. V. Lewis, D. V. Dawson, and R. Radtke. Temporal lobectomy and independent bitemporal interictal activity: What degree of lateralization is sufficient? Epilepsia 32:195-201, 1991.
Davey, B. L. K., W. R. Fright, G. J. Caroll, and R. D. Jones. Expert system approach to detection of epileptiform activity in the EEG. Med. Biol. Eng. Comput. 27:365-370, 1989.
Dingle, A. A., R. D. Jones, G. J. Caroll, and W. R. Fright. A multistage system to detect epileptiform activity in the EEG. IEEE Trans. Biomed. Eng. 40:1260-1268, 1993.
Dreyfus, H. L., and S. E. Dreyfus. Mind Over Machine. New York: The Free Press, 1985.
Gabor, A. J., and M. Seyal. Automated interictal EEG spike detection using artificial neural networks. Electroencepha-logr. Clin. Neurophysiol. 83:271-280, 1992.
Gilliam, F., S. Bowling, E. Bilir, J. Thomas, E. Faught, R. Morawetz, C. Palmer, J. Hugg, and R. Kuzniecky. Association of combined MRI, interictal EEG and ictal EEG results with outcome and pathology after temporal lobectomy. Epilepsia 38:1315-1320, 1998.
Glover, Jr., J. R., P. Y. Ktonas, N. Raghavan, J. M. Urunñela, S. S. Velamuri, and E. L. Reilly. A multichannel signal processor for the detection of epileptogenic sharp transients in the EEG. IEEE Trans. Biomed. Eng. 33:1121-1128, 1986.
Glover, Jr., J. R., N. Raghavan, P. Y. Ktonas, and J. D. Frost, Jr. Context-based automated detection of epileptogenic sharp transients in the EEG: Elimination of false positives. IEEE Trans. Biomed. Eng. 36:519-527, 1989.
Godoy, J., H. Lüders, D. S. Dinner, H. H. Morris III., E. Wyllie, and D. Murphy. Significance of sharp waves in rou-tine EEGs after epilepsy surgery. Epilepsia 33:285-288, 1992.
Gotman, J., and P. Gloor. Automatic recognition and quanti-fication of interictal epileptic activity in the human scalp EEG. Electroencephalogr. Clin. Neurophysiol. 41:513-529, 1976.
Gotman, J., J. R. Ives, and R. Gloor. Automatic recognition of interictal epileptic activity in prolonged EEG recordings. Electroencephalogr. Clin. Neurophysiol. 46:510-520, 1979.
Gotman, J., and L. Y. Wang. State-dependent spike detection: Concepts and preliminary results. Electroencephalogr. Clin. Neurophysiol. 79:11-19, 1991.
Gotman, J., and L. Y. Wang. State-dependent spike detection: Validation. Electroencephalogr. Clin. Neurophysiol. 83:12-18, 1992.
Jando, G., R. M. Siegel, Z. Horvath, and G. Buzsaki. Pattern recognition of the electroencephalogram by artificial neural networks. Electroencephalogr. Clin. Neurophysiol. 86:100-109, 1993.
Kanner, A. M., H. H. Morris III., H. O. Lüders, D. S. Dinner, P. Van Ness, and E. Wyllie. Usefulness of unilateral interictal sharp waves of temporal lobe origin in prolonged video-EEG monitoring studies. Epilepsia 34:884-889, 1993.
Kohonen, T. Analysis of a simple self-organizing process. Biol. Cybern. 40:135-140, 1982.
Kohonen, T. Self-Organization and Associative Memory. New York: Springer, 1984.
Kohonen, T. The self-organizing map. Proc. IEEE 78:1464-1480, 1990.
Kohonen, T. Self-Organizing Maps, 2nd ed. New York: Springer, 1997.
Morris III., H. H., A. Kanner, H. O. Lüders, D. Murphy, D. S. Dinner, E. Wyllie, and P. Kotagal. Sharp waves at the sphenoidal electrode accurately identify a mesio-temporal epileptogenic focus? Epilepsia 30:532-539, 1989.
Özdamar, O., I. Yaylali, P. Jayakar, and C. N. Lopez. Multilevel neural network system for EEG spike detection. In: Computer-Based Medical Systems. Proceedings of the 4th Annual IEEE Symposium, edited by I. N. Bankmann and J. E. Tsitlik. Washington, DC: IEEE Computer Society, 1991, pp. 272-279.
Peltoranta, M., and G. Pfurtscheller. Neural network-based classification of nonaveraged event-related EEG responses. IIG Report No. 328, 1992.
Pilcher, W. H., D. W. Roberts, H. F. Flanigin, P. H. Crandall, H. G. Wieser, G. A. Ojemann, and W. J. Peacock. Complications of epilepsy surgery. In: Surgical Treatment of the Epilepsies, 2nd ed., edited by J. Engel, Jr. New York: Raven, 1993, pp. 565-581.
Risinger, M. W., J. Engel, Jr., P. C. Van Ness, T. R. Henry, and P. H. Crandall. Ictal localization of temporal lobe seizures with scalp/sphenoidal recordings. Neurology 39:1288-1293, 1989.
27 Rumelhart, D. E., G. E. Hinton, and R. J. Williams. Learning internal representation by error propagation. In: Parallel Distributed Processing, Vol. I, edited by D. E. Rumelhard and J. L. McCalland. Cambridge, MA: MIT, 1986.
Spatt, J., G. Pelzl, and B. Mamoli. Reliability of automatic and visual analysis of interictal spikes in lateralizing an epileptic focus during video-EEG monitoring. Electroencephalogr. Clin. Neurophysiol. 103:421-425, 1997.
29 Steinhoff, B. J., N. K. So, S. Lim, and H. O. Lüders. Ictal scalp EEG in temporal lobe epilepsy with unitemporal versus bitemporal interictal epileptiform discharges. Neurology 45:889-896, 1995.
Ultsch, A. Self-organized feature maps for monitoring and knowledge acquisition of a chemical process. Proceedings of the International Conference on Artificial Neural Networks, 1993 (unpublished).
Webber, W. R. S., B. Litt, R. P. Lesser, R. S. Fisher, and I. Bankman. Automatic EEG spike detection: what should the computer imitate. Electroencephalogr. Clin. Neurophysiol. 87:364-373, 1993.
Webber, W. R. S., B. Litt, K. Wilson, and R. P. Lesser. Practical detection of epileptiform discharges (EDs) in the EEG using an artificial neural network: A comparison of raw and parameterized EEG data. Electroencephalogr. Clin. Neurophysiol. 91:194-204, 1994.
Wilson, K., W. R. S. Webber, R. P. Lesser, R. S. Fisher, R. C. Eberhart, and R. W. Dobbins. Detection of epileptiform spikes in the EEG using a patient-independent neural net-work. In: Computer-Based Medical Systems. Proceedings of the 4th IEEE Symposium, edited by I. N. Bankmann and J. E. Tsitlik. Washington, DC: IEEE Computer Society, 1991, pp. 264-271.
Wilson, S. B., R. N. Harner, B. R. Duffy, B. R. Tharp, M. R. Nuwer, and M. R. Sperling. Spike detection. I. Correlation and reliability of human experts. Electroencephalogr. Clin. Neurophysiol. 98:186-198, 1996.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kurth, C., Gilliam, F. & Steinhoff, B.J. EEG Spike Detection With a Kohonen Feature Map. Annals of Biomedical Engineering 28, 1362–1369 (2000). https://doi.org/10.1114/1.1331312
Issue Date:
DOI: https://doi.org/10.1114/1.1331312