Summary
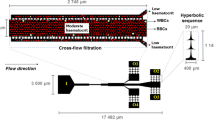
An in-house fabricated microfluidic channel was developed using easily available materials (glass slide, transparency shapes, polyvinyl chloride tubes, coverslip, and epoxy) and manual positioning techniques with the aid of a profile projector, in the absence of other fabrication facilities.2,3,11–15 Despite the limitations of being less accurate and more laborious, manual methods offer an easy, inexpensive, and readily implementable alternative to automated ones. Being economical, they enable testing of newer models and hypothesis, development of new designs, and trail stage/prototype experimentation. Once the effectiveness of the developed channel is established, the manual fabrication method can eventually be automated. So far as erythrocyte deformability is concerned, the microchannel thus fabricated enabled multishape erythrocyte deformability analysis in contrast to the unishape approach by earlier studies.11–15,19 Though the microchannel in this study has larger width (30 μm) compared to the mean RBC diameter (~8 μm), it allows free orientation of cells and larger variation of shear condition across the channel cross section. Consequently, both asymmetrical and axial shapes were observed during the flow. Hence, it may be concluded that a larger cross section and medium to high flow (10–20 mm/s) may be appropriate for multishape characterization of erythrocytes in dilute suspensions. The issue of determining the DI for several deformed shapes and sorting them from other interacting and nondeforming RBCs could be handled with more flexible software techniques.
Similar content being viewed by others
References
Beebe, D., and Folch, A., “The Science and Applications of Cell Biology in Microsystems,” Lab On A Chip, 5:10–11 (2005).
Sia, S.K., and Whitesides, G.M., “Microfluidic Devices Fabricated in Poly(dimethylsiloxane) for Biological Studies,” Electrophoresis, 24:3563–3576 (2003).
McDenold, J.C., and Whitesides, G.M., “Poly(dimethylsiloxane) as a Material for Fabricating Microfluidic Devices,” Accounts of Chemical Research, 35(7):491–499 (2002).
Stoltz, J.F., Singh, M., and Riha, P., Hemorheology in Practice, IOS Press, Amsterdam, The Netherlands (1999).
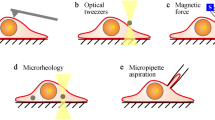
Jayavanth, S., and Park, B.C., “Microrheological Dysfunctions in Blood during Malaria,” Indian Journal of Experimental Biology, 45:111–120 (2007).
Chien, S., “Red Cell Deformability and its Relevance to Blood Flow,” Annual Review of Physiology, 49:177–192 (1987).
Mohrman, D.E., and Heller, L.J., Cardiovascular Physiology, 3rd Edition, McGraw-Hill, Inc., New York (1991).
Chien, S., “Biophysical Behavior of Red Cells in Suspension,” Surgenor, D.M. (ed), The Red Blood Cell, Academic, New York, pp. 1032–1133 (1975).
Schmid-Schönbein, H., and Grunau, G., The Flow Behaviour of Blood (Exempla Haemorheologica 2), Hoechst, Frankfurt, Germany (1980).
Mchedlishvili, G., “Disturbed Blood Flow Structuring as Critical Factor of Hemorheological Disorders in Microcirculation,” Clinical Hemorheology Microcirculation, 19:315–325 (1998).
Kikuchi, Y., Ohki, H., Kaneko, T., and Sato, K., “Optically Accessible Microchannels Formed in a Single Crystal Silicon Substrate for Studies of Blood Rheology,” Microvascular Research, 44:226–240 (1992).
Tan, A., Rodgers, K., Murrihy, J., O’Mathuna, C., and Glennon, J.D., “Rapid Fabrication of Microfluidic Devices in Polydimethylsiloxane by Photocopying,” Lab on Chip, 1(1):7–9 (2001).
Cokelet, G.R., Soave, R., Pugh, G., and Rathbun, L., “Fabrication of In Vitro Microvascular Blood Flow Systems by Photolithography,” Microvascular Research 46:394–400 (1993).
Sutton, N., Tracey, M.C., Johnston, I.D., Greenaway, R.S., and Rampling, M.W., A Novel Instrument for Studying the Flow Behaviour of Erythrocytes through Microchannels Simulating Human Blood Capillaries, Microvascular Research 53:272–281 (1997).
Tracey, M.C., Greenaway, R.S., Das, A., Kaye, P.H., and Barnes, A.J., “A Silicon Micromachined Device for Use in Blood Cell Deformability Studies,” IEEE Transactions on Biomedical Engineering 42:751–761 (1995).
Jayavanth, S., and Singh, M., “Computerized Analysis of Erythrocyte Aggregation from Sequential Video-Microscopic Images under Gravitational Sedimentation,” Innovation and Technology in Medicine and Biology 25:61–68 (2004).
Shelby, J.P., White, J., Ganesan, K., Rathod, P.K., and Chiu Daniel, T., “A Microfluidic Model for Single-Cell Capillary Obstruction by Plasmodium Falciparum Infected Erythrocytes,” Proceedings of the National Academy of Sciences, 100(25):14618–14622 (2003).
Faivre, M., Abkarian, M., Bickraj, K., and Stone Howard, A., “Geometrical Focusing of Cells in a Microfluidic Device: An Approach to Separate Blood Plasma,” Biorheology 43:147–159 (2006).
Tsukada, K., Sekizuka, E., Oshio, C., and Minamitani, H., “Direct Measurement of Erythrocyte Deformability in Diabetes Mellitus with a Transparent Microchannel Capillary Model and HighSpeed Video Camera System,” Microvascular Research 61:231–239 (2001).
Goldsmith, H.L., and Marlow, J., “Flow Behaviour of Erythrocytes 1. Rotation and Deformation in Dilute Suspensions,” Proceedings of the Royal Society of London, Series B, 182:351–384 (1972).
Gaehtgens, P., Schmidt, F., and Will, G., “Comparative Rheology of Nucleated and Non-Nucleated Red Blood Cells,” Pflugers Archiv 390:278–282 (1981).
Jeong, J.H., Sugii, Y., Minamiyama, M., Okamoto, K., “Measurement of RBC Deformation and Velocity in Capillaries In Vivo,” Microvascular Research 71(3):212–218 (2006).
Schmid-Schonbein, H., Wells, R., and Schildkraut, R., “Microscopic and Viscometry of Blood Flowing under Uniform Shear Rate (Rheoscope),” Journal of Applied Physiology 26:674–678 (1969).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayavanth, S., Lee, D.H. & Pak, B.C. A microchannel for in vitro imaging of erythrocyte shape transformations by video microscopic technique. Exp Tech 33, 15–20 (2009). https://doi.org/10.1111/j.1747-1567.2008.00397.x
Published:
Issue Date:
DOI: https://doi.org/10.1111/j.1747-1567.2008.00397.x