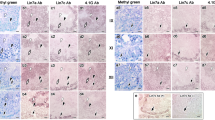
Abstract
The polarized architecture of epithelial cells is a fundamental determinant of cell structures and functions. Both formation and orientation of proper epithelial polarity are needed for cell-cell or cell-matrix adhesion, signal transduction and cytoskeletal interactions of multimolecular complexes at apical, lateral and basal cell membranes. These cell membrane domains are usually segregated by some junctional complexes. Recent molecular genetic studies on the anchor structure between myelin sheaths and axons have indicated the specific molecular organization for polarization of axolemma and the myelin sheaths at paranodes, termed ‘septate-like junctions’. It was also speculated that other mammalian organs may use a similar junctional system. The protein 4.1B was originally found to be localized in paranodes and juxtaparanodes of myelinated nerve fibers. Our recent immunohistochemical studies on protein 4.1B have indicated its significance for the cell-cell and/or cell-matrix adhesion in various rodent organs. The protein 4.1 family of proteins have been supposed to possess variable molecular domains relating to cell adhesion, ion balance, receptor responses and signal transduction. Therefore, more precise studies on the molecular structure and the functional domains of protein 4.1B, as well as on its changes under physiological and pathological conditions, may provide a clue for organogenesis in various mammalian organs.
Similar content being viewed by others
References
An XL, Takakuwa Y, Nunomura W, Manno S, Mohandas N (1996) Modulation of band 3-ankyrin interaction by protein 4.1. Functional implications in regulation of erythrocyte membrane mechanical properties. J Biol Chem 271, 33 187–91.
Bennett V, Gilligan DM (1993) The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol 9, 27–66.
Binda AV, Kabbani N, Lin R, Levenson R (2002) D2 and D3 dopamine receptor cell surface localization mediated by interaction with protein 4.1N. Mol Pharmacol 62, 507–13.
Borg JP, Marchetto S, Le Bivic A et al. (2000) ERBIN: A basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nat Cell Biol 2, 407–14.
Braga VM (2002) Cell-cell adhesion and signaling. Curr Opin Cell Biol 14, 546–56.
Branton D, Cohen CM, Tyler J (1981) Interaction of cytoskeletal protein on human erythrocyte membrane. Cell 24, 24–32.
Carboni JM, Howe CL, West AB, Barwick KW, Mooseker MS, Morrow JS (1987) Characterization of intestinal brush border cytoskeletal proteins of normal and neoplastic human epithelial cells. A comparison with the avian brush border. Am J Pathol 129, 589–600.
Cheng CY, Mruk DD (2002) Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev 82, 825–74.
Chishti AH, Kim AC, Marfatia SM et al. (1998) The FERM domain: A unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem Sci 23, 281–2.
Cho EA, Patterson LT, Brookhiser WT, Mah S, Kintner C, Dressler GR (1998) Differential expression and function of cadherin-6 during renal epithelium development. Development 125, 803–12.
Christofori G, Semb H (1999) The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem Sci 24, 73–6.
Cohen AR, Woods DF, Marfatia SM et al. (1998) Human CASK/ LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol 142, 129–38.
Coleman TR, Harris AS, Mische SM, Mooseker MS, Morrow JS (1987) Beta spectrin bestows protein 4.1 sensitivity on spectrin-actin interactions. J Cell Biol 104, 519–26.
Conboy J (1999) The role of alternative pre-mRNA splicing in regulating the structure and function of skeletal protein 4.1. Proc Soc Exp Biol Med 220, 73–8.
Correas I, Speicher DW, Marchesi VT (1986) Structure of the spectrin-actin binding site of erythrocyte protein 4.1. J Biol Chem 261, 13 362–6.
Deguchi M, Iizuka T, Hata Y et al. (2000) PAPIN. A novel multiple PSD-95/Dlg-A/ZO-1 protein interacting with neural plakophilin-related armadillo repeat protein/delta-catenin and p0071. J Biol Chem 275, 29 875–80.
Denisenko-Nehrbass N, Goutebroze L, Galvez T et al. (2003) Association of Caspr/paranodin with tumor suppressor schwannomin/merlin and β1 integrin in the central nervous system. J Neurochem 84, 209–21.
Denker SP, Barber DL (2002) Ion transport proteins anchor and regulate the cytoskeleton. Curr Opin Cell Biol 14, 214–20.
Dmytrenko GM, Pumplin DW, Bloch RJ (1993) Dystrophin in a membrane skeletal network: Localization and comparison to other proteins. J Neurosci 13, 547–58.
Einheber S, Zanazzi G, Ching W et al. (1997) The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J Cell Biol 139, 1495–506.
Elgsaeter A, Stokke BT, Mikkelsen A, Branton D (1986) The molecular basis of erythrocyte shape. Science 234, 1217–23.
Ervasti JM, Campbell KP (1993) Dystrophin and the membrane skeleton. Curr Opin Cell Biol 5, 82–7.
Feraille E, Doucet A (2001) Sodium-potassium-adenosine triphosphatase-dependent sodium transport in the kidney: Hormonal control. Physiol Rev 81, 345–418.
Fujiwara T, Ritchie K, Murakoshi H, Jacobson K, Kusumi A (2002) Phospholipids undergo hop diffusion in compartmentalized cell membrane. J Cell Biol 157, 1071–81.
Garcia-Alvarez B, de Pereda JM, Calderwood DA et al. (2003) Structural determinants of integrin recognition by talin. Mol Cell 11, 49–58.
Gimm JA, An X, Nunomura W, Mohandas N (2002) Functional characterization of spectrin-actin-binding domains in 4.1 family of proteins. Biochemistry 41, 7275–82.
Gollan L, Sabanay H, Poliak S, Berglund EO, Ranscht B, Peles E (2002) Retention of a cell adhesion complex at the paranodal junction requires the cytoplasmic region of Caspr. J Cell Biol 157, 1247–56.
Gross E, Hopfer U (1999) Effects of pH on kinetic parameters of the Na-HCO3 cotransporter in renal proximal tubule. Biophys J 76, 3066–75.
Gutmann DH, Hirbe AC, Huang ZY, Haipek CA (2001) The protein 4.1 tumor suppressor, DAL-1, impairs cell motility, but regulates proliferation in a cell-type-specific fashion. Neurobiol Dis 8, 266–78.
Hamada K, Shimizu T, Yonemura S, Tsukita S, Tsukita S, Hakoshima T (2003) Structural basis of adhesion-molecule recognition by ERM proteins revealed by the crystal structure of the radixin-ICAM-2 complex. EMBO J 22, 502–14.
Han BG, Nunomura W, Takakuwa Y, Mohandas N, Jap BK (2000) Protein 4.1R core domain structure and insights into regulation of cytoskeletal organization. Nat Struct Biol 7, 871–5.
Hanada T, Takeuchi A, Sondarva G, Chishti AH (2003) Protein 4.1-mediated membrane targeting of human discs large in epithelial cells. J Biol Chem 278, 34 445–50.
Hatzfeld M, Nachtsheim C (1996) Cloning and characterization of a new armadillo family member, p0071, associated with the junctional plaque: Evidence for a subfamily of closely related proteins. J Cell Sci 109, 2767–78.
Hiscox S, Jiang WG (1999) Ezrin regulates cell-cell and cell-matrix adhesion, a possible role with E-cadherin/beta-catenin. J Cell Sci 112, 3081–90.
Holzwarth G, Yu J, Steck TL (1976) Heterogeneity in the conformation of different protein fractions from the human erythrocyte membrane. J Supramol Struct 4, 161–8.
Izawa I, Nishizawa M, Tomono Y, Ohtakara K, Takahashi T, Inagaki M (2002) ERBIN associates with p0071, an armadillo protein, at cell-cell junctions of epithelial cells. Gene Cell 7, 475–85.
Jaulin-Bastard F, Arsanto JP, Le Bivic A et al. (2002) Interaction between Erbin and a Catenin-related protein in epithelial cells. J Biol Chem 277, 2869–75.
Kordeli E (2000) The spectrin-based skeleton at the postsynaptic membrane of the neuromuscular junction. Microsc Res Tech 49, 101–7.
Lee NP, Mruk D, Lee WM, Cheng CY (2003) Is the cadherin/ catenin complex a functional unit of cell-cell actin-based adherens junctions in the rat testis? Biol Reprod 68, 485–508.
Lee S, Baek M, Yang H et al. (2002) Identification of genes differentially expressed between gastric cancers and normal gastric mucosa with cDNA microarrays. Cancer Lett 184, 197–206.
Lombardo CR, Willardson BM, Low PS (1992) Localization of the protein 4.1-binding site on the cytoplasmic domain of erythrocyte membrane band 3. J Biol Chem 267, 9540–6.
Lu D, Yan H, Othman T, Turner CP, Woolf T, Rivkees SA (2004) Cytoskeletal protein 4.1G binds to the third intracellular loop of the A1 adenosine receptor and inhibits receptor action. Biochem J 377, 51–9.
Lui WY, Mruk DD, Lee WM, Cheng CY (2003) Adherens junction dynamics in the testis and spermatogenesis. J Androl 24, 1–14.
Luque CM, Lallena MJ, Alonso MA, Correas I (1998) An alternative domain determines nuclear localization in multifunctional protein 4.1. J Biol Chem 273, 11 643–9.
Marfatia SM, Lue RA, Branton D, Chishti AH (1994) In vitro binding studies suggest a membrane-associated complex between erythroid p55, protein 4.1, and glycophorin C. J Biol Chem 269, 8631–4.
Marfatia SM, Lue RA, Branton D, Chishti AH (1995) Identification of the protein 4.1 binding interface on glycophorin C and p55, a homologue of the Drosophila discs-large tumor suppressor protein. J Biol Chem 270, 715–19.
Martinez-Estrada OM, Villa A, Breviario F, Orsenigo F, Dejana E, Bazzoni G (2001) Association of junctional adhesion molecule with calcium/calmodulin-dependent serine protein kinase (CASK/LIN-2) in human epithelial caco-2 cells. J Biol Chem 276, 9291–6.
Matsumine A, Ogai A, Senda T et al. (1996) Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein. Science 272, 1020–203.
Mattagajasingh SN, Huang SC, Hartenstein JS, Benz EJ Jr (2000) Characterization of the interaction between protein 4.1R and ZO-2. J Biol Chem 29, 30 573–85.
Mohandas N, Chasis JA (1993) Red blood cell deformability, membrane material properties and shape: Regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin Hematol 30, 171–92.
Mulholland DJ, Dedhar S, Vogl AW (2001) Rat seminiferous epithelium contains a unique junction (ectoplasmic specialization) with signaling properties both of cell/cell and cell/matrix junctions. Biol Reprod 64, 396–407.
Nagase T, Ishikawa K, Nakajima D et al. (1997) Prediction of the coding sequences of unidentified human genes. VII. The complete sequences of 100 new cDNA clones from brain which can code for large proteins in vitro. DNA Res 4, 141–50.
Nagase T, Kikuno R, Ishikawa K, Hirosawa M, Ohara O (1999) Prediction of the coding sequences of unidentified human genes. XIII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res 6, 63–70.
Niggli V (2001) Structural properties of lipid-binding sites in cytoskeletal proteins. Trends Biochem Sci 26, 604–11.
Nix SL, Chishti AH, Anderson JM, Walther Z (2000) hCASK and hDlg associate in epithelia, and their src homology 3 and guanylate kinase domains participate in both intramolecular and intermolecular interactions. J Biol Chem 275, 41 192–200.
Nunomura W, Takakuwa Y, Parra M, Conboy J, Mohandas N (2000a) Regulation of protein 4.1R, 55, and glycophorin C ternary complex in human erythrocyte membrane. J Biol Chem 275, 24 540–6.
Nunomura W, Takakuwa Y, Parra M, Conboy JG, Mohandas N (2000b) Ca2+-dependent and Ca2+-independent calmodulin binding sites in erythrocyte protein 4.1. Implications for regulation of protein 4.1 interactions with transmembrane proteins. J Biol Chem 275, 6360–7.
Ohara R, Yamakawa H, Nakayama M, Ohara O (2000) Type II, 4.1 (4.1B/KIAA0987), a member of the protein 4.1 family, is localized to neuronal paranodes. Mol Brain Res 85, 41–52.
Ohno H, Hirabayashi S, Iizuka T, Ohnishi H, Fujita T, Hata Y (2002) Localization of p0071-interacting proteins, plakophilin-related armadillo-repeat protein-interacting protein (PAPIN) and ERBIN, in epithelial cells. Oncogene 21, 7042–9.
Ohno H, Hirabayashi S, Kansaku A et al. (2003) Carom: A novel membrane-associated guanylate kinase-interacting protein with two SH3 domains. Oncogene 22, 8422–31.
Ohno N, Terada N, Murata S et al. (2004) Immunolocalization of protein 4.1B/DAL-1 during neoplastic transformation of mouse and human intestinal epithelium. Histochem Cell Biol 122, 579–86.
Parra M, Gascard P, Walensky LD, Snyder SH, Mohandas N, Conboy JG (1998) Cloning and characterization of 4.1G (EPB41L2), a new member of the skeletal protein4.1 (EPB41) gene family. Genomics 49, 298–306.
Parra M, Gascard P, Walensky LD et al. (2000) Molecular and functional characterization of protein 4.1B, a novel member of the protein 4.1 family with high level, focal expression in brain. J Biol Chem 275, 3247–55.
Pasternak C, Wong S, Elson EL (1995) Mechanical function of dystrophin in muscle cells. J Cell Biol 128, 355–61.
Pearson MA, Reczek D, Bretscher A, Karplus PA (2000) Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 101, 259–70.
Perez-Moreno M, Jamora C, Fuchs E (2003) Sticky business: Orchestrating cellular signals at adherens junctions. Cell 112, 535–48.
Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G (1998) A casual role for E-cadherin in the transition from adenoma to carcinoma. Nature 392, 190–3.
Perl AK, Dahl U, Wilgenbus P, Cremer H, Semb H, Christofori G (1999) Reduced expression of neural cell adhesion molecule induces metastatic dissemination of pancreatic β tumor cells. Nat Med 5, 286–91.
Peters LL, Weier HU, Walensky LD et al. (1998) Four paralogous protein 4.1 genes map to distinct chromosomes in mouse and human. Genomics 54, 348–50.
Poliak S, Gollan L, Martinez R et al. (1999) Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+channels. Neuron 24, 1037–47.
Poliak S, Salomon D, Elhanany H et al. (2003) Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol 162, 1149–60.
Pradhan D, Lombardo CR, Roe S, Rimm DL, Morrow JS (2001) α-Catenin bands directly to spectrin and facilitates spectrin-membrane assembly in vivo. J Biol Chem 276, 4175–81.
Ramez M, Blot-Chabaud M, Cluzeaud F et al. (2003) Distinct distribution of specific members of protein 4.1 gene family in the mouse nephron. Kidney Int 63, 1321–37.
Reuver SM, Garner CC (1998) E-Cadherin mediated cell adhesion recruits SAP97 into the cortical cytoskeleton. J Cell Sci 111, 1071–80.
Saito H, Santoni MJ, Arsanto JP et al. (2001) Lano, a novel LAP protein directly connected to MAGUK proteins in epithelial cells. J Biol Chem 276, 32 051–5.
Sako Y, Kusumi A (1995) Barriers for lateral diffusion of transferrin receptor in the plasma membrane as characterized by receptor dragging by laser tweezers: Fence versus tether. J Cell Biol 129, 1559–74.
Shimizu T, Seto A, Maita N et al. (2002) Structural basis for neurofibromatosis type 2. Crystal structure of the merlin FERM domain. J Biol Chem 277, 10 332–6.
Smith WJ, Nassar N, Bretscher A, Cerione RA, Karplus PA (2003) Structure of the active N-terminal domain of Ezrin. Conformational and mobility changes identify keystone interactions. J Biol Chem 278, 4949–56.
Steck LS (1974) The organization of proteins in the human red blood cell membrane. J Cell Biol 62, 1–19.
Sun CX, Robb VA, Gutmann DH (2002) Protein 4.1 tumor suppressors: Getting a FERM grip on growth regulation. J Cell Sci 115, 3991–4000.
Takakuwa Y (2000) Protein 4.1, a multifunctional protein of the erythrocyte membrane skeleton: Structure and functions in erythrocytes and nonerythroid cells. Int J Hematol 72, 298–309.
Terada N, Ohno S (1998) Dynamic morphology of erythrocytes revealed by cryofixation technique. Acta Anat Nippon 73, 587–93.
Terada N, Fujii Y, Ueda H, Ohno S (1997) An immunocytochemical study of changes in the human erythrocyte membrane skeleton produced by stretching examined by the quickfreezing and deep-etching method. J Anat 190, 397–404.
Terada N, Ohno N, Yamakawa H et al. (2003) Protein 4.1B in mouse islets of Langerhans and β-cell tumorigenesis. Histochem Cell Biol 120, 277–83.
Terada N, Ohno N, Yamakawa H et al. (2004a) Immunolocalization of protein 4.1 B in the rat digestive system. J Mol Histol 35, 347–53.
Terada N, Ohno N, Yamakawa H et al. (2004b) Immunoelectron microscopic localization of protein 4.1B in proximal S1 and S2 tubules of rodent kidneys. Med Electron Microsc 37, 45–51.
Terada N, Ohno N, Yamakawa H et al. (2004c) Immunohistochemical study of protein 4.1 B in the normal and W/Wv mouse seminiferous epithelium. J Histochem Cytochem 52, 769–77.
Terada N, Ohno N, Yamakawa H et al. (2004d) Protein 4.1B localizes on unmyelinated axonal membranes in the mouse enteric nervous system. Neurosci Lett 366, 15–17.
Tomishige M, Sako Y, Kusumi A (1998) Regulation mechanism of the lateral diffusion of band 3 in erythrocyte membranes by the membrane skeleton. J Cell Biol 142, 989–1000.
Tran YK, Bogler O, Gorse KM, Wieland I, Green MR, Newsham IF (1999) A novel member of the NF2/ERM/4.1 superfamily with growth suppressing properties in lung cancer. Cancer Res 59, 35–43.
Uchida Y, Ogata M, Mori Y, Oh-hora M, Hatano N, Hamaoka T (2002) Localization of PTP-FERM in nerve processes through its FERM domain. Biochem Biophys Res Commun 22, 13–19.
von Ruckmann B, Jons T, Dolle F, Drenckhahn D, Schubert D (1997) Cytoskeleton-membrane connections in the human erythrocyte membrane: Band 4.1 binds to tetrameric band 3 protein. Biochim Biophys Acta 1325, 226–34.
Walensky LD, Blackshaw S, Liao D et al. (1999) A novel neuron-enriched homolog of the erythrocyte membrane cytoskeletal protein 4.1. J Neurosci 19, 6457–67.
Waugh R, Evans EA (1979) Thermoelasticity of red blood cell membrane. Biophys J 26, 115–31.
Workman RF, Low PS (1998) Biochemical analysis of potential sites for protein 4.1-mediated anchoring of the spectrin-actin skeleton to the erythrocyte membrane. J Biol Chem 273, 6171–6.
Yageta M, Kuramochi M, Masuda M et al. (2002) Direct association of TSLC1 and DAL-1, two distinct tumor suppressor proteins in lung cancer. Cancer Res 62, 5129–33.
Yamakawa H, Ohara O (2000) Comparison of mRNA and protein levels of four members of the protein 4.1 family: The type II brain 4.1/4.1B/KIAA0987 is the most predominant member of the protein 4.1 family in rat brain. Gene 248, 137–45.
Yamakawa H, Ohara R, Nakajima D, Nakayama M, Ohara O (1999) Molecular characterization of a new member of the protein 4.1 family (brain 4.1) in rat brain. Brain Res Mol Brain Res 70, 197–209.
Ye K, Compton DA, Lai MM, Walensky LD, Snyder SH (1999) Protein 4.1 N binding to nuclear mitotic apparatus protein in PC12 cells mediates the antiproliferative actions of nerve growth factor. J Neurosci 19, 10747–56.
Yonemura S, Hirao M, Doi Y et al. (1998) Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J Cell Biol 140, 885–95.
Yu T, Robb VA, Singh V, Gutmann DH, Newsham IF (2002) The 4.1/ezrin/radixin/moesin domain of the DAL-1/Protein 4.1B tumour suppressor interacts with 14-3-3 proteins. Biochem J 365, 783–9.
Zhang S, Mizutani A, Hisatsune C et al. (2003) Protein 4.1 N is required for translocation of inositol 1,4,5-trisphosphate receptor type 1 to the basolateral membrane domain in polarized Madin-Darby canine kidney cells. J Biol Chem 278, 4048–56.
Zimmermann P, Tomatis D, Rosas M et al. (2001) Characterization of syntenin, a syndecan-binding PDZ protein, as a component of cell adhesion sites and microfilaments. Mol Biol Cell 12, 339–50.
Zubrzycka-Gaarn EE, Bulman DE, Karpati G et al. (1988) The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature 333, 466–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Terada, N., Ohno, N., Yamakawa, H. et al. Topographical significance of membrane skeletal component protein 4.1B in mammalian organs. Anato Sci Int 80, 61–70 (2005). https://doi.org/10.1111/j.1447-073x.2005.00094.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1111/j.1447-073x.2005.00094.x