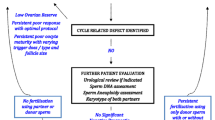
Abstract
Intracytoplasmic sperm injection (ICSI) is the most effective treatment for achieving fertilization in assisted reproductive technology (ART). However, fertilization failure occurs. The incidence of fertilization failure after ICSI is 1–5%. Approximately 50% of fertilization failure cases could be attributed to the abnormality of sperm factor. As the fertilization fails after ICSI using mature sperm, round spermatids and globozoospermia, artificial oocyte activation may provide a means of improving fertilization rates in such cases. The oocyte activation treatments used in clinical research include calcium (Ca) ionophore treatment, electrostimulation and strontium treatment. In terms of the efficiency of oocyte activation, electrostimulation and Ca ionophore gave better outcomes than strontium treatment. Strontium treatment causes Ca2+ oscillations in mice, so it has been viewed favorably. However, in human oocytes calcium oscillation has not been observed. The fertilization rate after ICSI was low in the case of globozoospermia and wiht round spermatids. Some cases of pregnancy were achieved by ICSI alone and oocyte activation methods were not essential in these cases. Among the various oocyte activation methods currently used, it should be noted that issues of genetic safety have not been addressed for the combined use of these oocyte activation methods.
Similar content being viewed by others
References
Carroll J. The initiation and regulation of Ca2+ signalling at fertilization in mammals. Seminars Cell Dev Biol 2001; 12: 37–43.
Schuetz AW. Cytoplasmic activation of starfish oocytes by sperm and divalent ionophore A-23187. J Cell Biol 1975; 66: 86–94.
Steinhardt RA, Epel D, Carroll EJ Jr, Yanagimachi R. Is calcium ionophore a universal activator for unfertilised eggs? Nature 1974; 252: 41–43.
Wakayama T, Perry ACF, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 1998; 394: 369–374.
Bradshaw J, Jung T, Fulka J Jr, Moor RM. UV irradiation of chromosomal DNA and its effect upon MPF and meiosis in mammalian oocytes. Mol Reprod Dev 1995; 41: 503–512.
Mahowald AP, Goralski TJ, Caulton JH. In vitro activation of Drosophila eggs. Dev Biol 1983; 98: 437–445.
Prather RS, Eichen PA, Nicks DK, Peters MS. Artificial activation of porcine oocytes matured in vitro. Mol Reprod Dev 1991; 28: 405–409.
Whittingham DG, Siracusa G. The involvement of calcium in the activation of mammalian oocytes. Exp Cell Res 1978; 113: 311–317.
Siracusa G, Whittingham DG, Molinaro M, Vivarelli E. Parthenogenetic activation of mouse oocytes induced by inhibitors of protein synthesis. J Embryol Exp Morphol 1978; 43: 157–166.
Hoshi K, Yanagida K, Sato A. Pretreatment of hamster oocytes with Ca2+ ionophore to facilitate fertilization by ooplasmic micro injection. Hum Reprod 1992; 7: 1992.
Yanagida K, Katayose H, Hoshi K, Yazawa H, Sato A. Effect of electrical stimulation on oocyte activation after intracytoplasmic sperm injection. J Mamm Ova Res 1997; 14: 132–138.
Tesarik J, Testart J. Treatment of sperm-injected human oocytes with Ca2+ ionophore supports the development of Ca2+ oscillations. Biol Reprod 1994; 51: 385–391.
Miyazaki S, Shirakawa H, Nakada H, Honda Y. Essential role of the inositol 1,4,5-triphosphate receptor/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev Biol 1993; 158: 62–78.
Swann K. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development 1990; 110: 1295–1302.
Stice SL, Robl JM. Activation of mammalian oocytes by a factor obtained from rabbit sperm. Mol Reprod Dev 1990; 25: 272–280.
Tesarik J, Sousa M, Testart J. Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod 1994; 9: 511–518.
Rybouchkin A, Dozortsev D, de Sutter P, Qian C, Dhont M. Intracytoplasmic injection of human spermatozoa into mouse oocytes: a useful model to investigate the oocyteactivating capacity and the karyotype of human spermatozoa. Hum Reprod 1995; 10: 1130–1135.
Araki Y, Yoshizawa M, Abe H, Murase Y, Araki Y. Use of mouse oocytes to evaluate the ability of human sperm to activate oocytes after failure of activation by intracytoplasmic sperm injection. Zygote 2004; 12: 111–116.
Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD, eds. The Physiology of Reproduction, 2nd edn. Raven Press, New York, 1994; 189–317.
Moor RM. Regulation of the meiotic cycle in oocytes of domestic mammals. Ann NY Acad Sci 1988; 541: 248–258.
Jones K, Carroll J, Merriman J, Whittingham D, Kono T. Repetitive sperm-induced Ca2+ transients in mouse oocytes are cell cycle dependent. Development 1995; 121: 3259–3266.
Bos-Mikich A, Whittingham DG, Jones KT. Meiotic and mitotic Ca2+ oscillations affect cell composition in resulting blastocysts. Dev Biol 1997; 182: 172–179.
Lawrence Y, Ozil J, Swann K. The effects of a Ca2+ chelator and heavy-metal-ion chelators upon Ca2+ oscillations and activation at fertilization in mouse eggs suggest a role for repetitive Ca2+ increases. Biochem J 1998; 335: 335–342.
Gordo A, Rodrigues P, Kurokawa M et al. Intracellular calcium oscillations signal apoptosis rather than activation in in vitro aged mouse eggs. Biol Reprod 2002; 66: 1828–1837.
Ozil J, Huneau D. Activation of rabbit oocytes: the impact of the Ca2+ signal regime on development. Development 2001; 128: 917–928.
Gerhart J, Wu M, Kirschner M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol 1984; 98: 1247–1255.
Hoshi K, Yanagida K, Yazawa H, Katayose H, Sato A. Intracytoplasmic sperm injection using immobilized or motile human spermatozoon. Fertil Steril 1995; 63: 1241–1245.
Yanagida K, Katayose H, Yazawa H et al. Successful fertilization and pregnancy following ICSI and electrical oocyte activation. Hum Reprod 1999; 14: 1307–1311.
Yanagida K, Morozumi K, Katayose H, Sato A. Successful pregnancy after ICSI with strontium oocyte activation in low rates of fertilization. Reprod Biomed Online 2006; 13: 2006.
Murase Y, Araki Y, Mizuno S et al. Pregnancy following chemical activation of oocytes in a couple with repeated failure of fertilization using ICSI: case report. Hum Reprod 2004; 19: 1604–1607.
Navara CS, First NL, Schatten G. Microtubule organization in the cow during fertilization, polyspermy, parthenogenesis, and nuclear transfer: the role of the sperm aster. Dev Biol 1994; 162: 29–40.
Eldar-Geva T, Brooks B, Margalioth EJ, Zylber-Haran E, Gal M, Silber SJ. Successful pregnancy and delivery after calcium ionophore oocyte activation in a normozoospermic patient with previous repeated failed fertilization after intracytoplasmic sperm injection. Fertil Steril Supplement 2003; 3: 1656–1658.
Chi HJ, Koo JJ, Song SJ, Lee JY, Chang SS. Successful fertilization and pregnancy after intracytoplasmic sperm injection and oocyte activation with calcium ionophore in a normozoospermic patient with extremely low fertilization rates in intracytoplasmic sperm injection cycles. Fertil Steril 2004; 82: 475–477.
Heindryckx B, Van der Elst J, De Sutter P, Dhont M. Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI. Hum Reprod 2005; 20: 2237–2241.
Ahmady A, Michael E. Successful pregnancy and delivery following intracytoplasmic injection of frozen-thawed nonviable testicular sperm and oocyte activation with calcium ionophore. J Androl 2007; 28: 13–14.
Nasr-Esfahani MH, Razavi S, Javdan Z, Tavalaee M. Artificial oocyte activation in severe teratozoospermia undergoing intracytoplasmic sperm injection. Fertil Steril 2008; 16 [Epub ahead of print].
Swann K, Ozil JP. Dynamics of the calcium signal that triggers mammalian egg activation. Int Rev Cytol 1994; 152: 183–222.
Zhang J, Wang CW, Blaszcyzk A et al. Electrical activation and in vitro development of human oocytes that fail to fertilize after intracytoplasmic sperm injection. Fertil Steril 1999; 72: 509–512.
Zimmerman U, Vienken J. Electric field-induced cell-to-cell fusion. J Membr Biol 1982; 67: 165–182.
Bates GW, Saunders A, Sowers AE. Electrofusion. In: Sowers AE, ed. Cell Fusion. Plenum Press, New York, 1987; 367–395.
Ozil JP. The parthenogenetic development of rabbit oocytes after repetitive pulsatile electrical stimulation. Development 1990; 109: 117–127.
Cheek TR, McGuinness OM, Vincent C, Moreton RB, Berridge MJ, Johnson MH. Fertilisation and thimerosal stimulate similar calcium spiking patterns in mouse oocytes but by separate mechanisms. Development 1993; 119: 179–189.
Kato M, Ishikawa A, Hochi S, Hirabayashi M. Effect of activation regimens for rat oocytes on full-term development after round spermatid injection. Contemp Top Lab Anim Sci 2004; 43: 13–15.
Tateno H, Kamiguchi Y. Parthenogenetic activation of Chinese hamster oocytes by chemical stimuli and its cytogenetic evaluation. Mol Reprod Dev 1997; 47: 72–78.
Méo SC, Yamazaki W, Leal CL, de Oliveira JA, Garcia JM. Use of strontium for bovine oocyte activation. Theriogenology 2005; 63: 2089–2102.
Okada K, Miyano T, Miyake M. Activation of pig oocytes by intracytoplasmic injection of strontium and barium. Zygote 2003; 11: 159–165.
Kono T, Jones KT, Mikich AB, Whittingham DG, Carroll J. A cell cycle-associated change in Ca2+ releasing activity leads to the generation of Ca2+ transients in mouse embryos during the first mitotic division. J Cell Biol 1996; 132: 915–923.
Zhang D, Pan L, Yang LH, He XK, Huang XY, Sun FZ. Strontium promotes calcium oscillations in mouse meiotic oocytes and early embryos through InsP3 receptors, and requires activation of phospholipase and the synergistic action of InsP3. Hum Reprod 2005; 20: 3053–3061.
Morozumi K, Tateno H, Yanagida K, Katayose H, Kamiguchi Y, Sato A. Chromosomal analysis of mouse spermatozoa following physical and chemical treatments that are effective in inactivating HIV. Zygote 2004; 12: 339–344.
Moomjy M, Sills ES, Rosenwaks Z, Palermo GD. Implications of complete fertilization failure after intracytoplasmic sperm injection for subsequent fertilization and reproductive outcome. Hum Reprod 1998; 13: 2212–2216.
Ludwig M, Strik D, Al-Hasani S, Diedrich K. No transfer in a planned ICSI cycle: we cannot overcome some basic rules of human reproduction. Eur J Obstet Gynecol Reprod Biol 1999; 87: 3–11.
Yanagida K. Complete fertilization failure in ICSI. Hum Cell 2004; 17: 187–193.
Liu J, Nagy Z, Joris H et al. Analysis of 76 total fertilization failure cycles out of 2732 intracytoplasmic sperm injection cycles. Hum Reprod 1995; 10: 2630–2636.
Esfandiari N, Javed MH, Gotlieb L, Casper RF. Complete failed fertilization after intracytoplasmic sperm injection — analysis of 10 years’ data. Int J Fertil Womens Medical 2005; 50: 187–192.
Saunders CM, Larman MG, Parrington J. et al. PLC zeta: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 2002; 129: 3533–3544.
Kuvist U. Importance of spermatozoal zinc as temporary inhibitor of sperm nuclear chromatin decondensation ability in man. Acta Phisiol Scand 1980; 109: 79–84.
Terada Y, Nakamura S, Simerly C et al. Centrosomal function assessment in human sperm using heterologous ICSI with rabbit eggs: a new male factor infertility assay. Mol Reprod Dev 2004; 67: 360–365.
Yamano S, Nakagawa K, Nakasaka H, Aono T. Fertilization failure and oocyte activation. J Med Invest 2000; 47: 1–8.
Nakagawa K, Yamano S, Moride N, Yamashita M, Yoshizawa M, Aono T. Effect of activation with Ca ionophore A23187 and puromycin on the development of human oocytes that failed to fertilize after intracytoplasmic sperm injection. Fertil Steril 2001; 76: 148–152.
Tesarik J, Rienzi L, Ubaldi F, Mendoza C, Greco E. Use of a modified intracytoplasmic sperm injection technique to overcome sperm-borne and oocyte-borne oocyte activation failures. Fertil Steril 2002; 78: 619–624.
Ebner T, Moser M, Sommergruber M, Jesacher K, Tews G. Complete oocyte activation failure after ICSI can be overcome by a modified injection technique. Hum Reprod 2004; 19: 1837–1841.
Lu Q, Zhao Y, Gao X et al. Combination of calcium ionophore A23187 with puromycin salvages human unfertilized oocytes after ICSI. Eur J Obstet Gynecol Reprod Biol 2006; 126: 72–76.
Manipalviratn S, Ahnonkitpanit V, Numchaisrika P, Chompurat D, Pansatha J, Suwajanakorn S. Results of direct current electrical activation of failed-to-fertilize oocytes after intracytoplasmic sperm injection. J Reprod Med 2006; 51: 493–499.
Moaz MN, Khattab S, Foutouh IA, Mohsen EA. Chemical activation of oocytes in different types of sperm abnormalities in cases of low or failed fertilization after ICSI: a prospective pilot study. Reprod Biomed Online 2006; 13: 791–794.
Kullander S, Rausing A. On round-headed human spermatozoa. IntJ Fertil 1975; 20: 33–40.
Liu J, Nagy Z, Joris H, Tournaye H, Devroey P, Van Steirteghem A. Successful fertilization and establishment of pregnancies after intracytoplasmic sperm injection in patients with globozoospermia. Hum Reprod 1995; 10: 626–629.
Trokoudes KM, Danos N, Kalogirou L et al. Pregnancy with spermatozoa from a globozoospermic man after intracytoplasmic sperm injection treatment. Hum Reprod 1995; 10: 880–882.
Bourne H, Liu DY, Clarke GN, Baker HW. Normal fertilization and embryo development by intracytoplasmic sperm injection of round-headed acrosomeless sperm. Fertil Steril 1995; 63: 1329–1332.
Battaglia DE, Koehler JK, Klein NA, Tucker MJ. Failure of oocyte activation after intracytoplasmic sperm injection using round-headed sperm. Fertil Steril 1997; 68: 118–122.
Rybouchkin A, Van Der Elst J, De Sutter P, Dhont M. ‘Globe-headed spermatozoa’ and ICSI. Fertil Steril 1998; 69: 361–362.
Stone S, O’Mahony F, Khalaf Y, Taylor A, Braude P. A normal livebirth after intracytoplasmic sperm injection for globozoospermia without assisted oocyte activation: case report. Hum Reprod 2000; 15: 139–141.
Kim ST, Cha YB, Park JM, Gye MC. Successful pregnancy and delivery from frozen-thawed embryos after intracytoplasmic sperm injection using round-headed spermatozoa and assisted oocyte activation in a globozoospermic patient with mosaic Down syndrome. Fertil Steril 2001; 75: 445–447.
Zeyneloglu HB, Baltaci V, Duran HE, Erdemli E, Batioglu S. Achievement of pregnancy in globozoospermia with Y chromosome microdeletion after ICSI. Hum Reprod 2002; 17: 1833–1836.
Nardo LG, Sinatra F, Bartoloni G, Zafarana S, Nardo F. Ultrastructural features and ICSI treatment of severe teratozoospermia: report of two human cases of globozoospermia. Eur J Obstet Gynecol Reprod Biol 2002; 104: 40–42.
Kilani Z, Ismail R, Ghunaim S et al. Evaluation and treatment of familial globozoospermia in five brothers. Fertil Steril 2004; 82: 1436–1439.
Khalili MA, Kalantar SM, Vahidi S, Ghafour-Zadeh M. Failure of fertilization following intracytoplasmic injection of round-headed sperm. Ann Saudi Med 1998; 18: 408–411.
Dirican EK, Isik A, Vicdan K, Sozen E, Suludere Z. Clinical pregnancies and livebirths achieved by intracytoplasmic injection of round headed acrosomeless spermatozoa with and without oocyte activation in familial globozoospermia: case report. Asian J Androl 2007: [Epub ahead of print].
Tesarik J, Mendoza C, Testart J. Viable embryos from injection of round spermatids into oocytes. N Engl J Med 1995; 333: 525.
Tesarik J, Mendoza C. Spermatid injection into human oocytes. I. Laboratory techniques and special features of zygote development. Hum Reprod 1996; 11: 772–779.
Tanaka A, Nagayoshi M, Awata S et al. Clinical evaluation of round spermatid injection (ROSI) into human oocytes. Fertil Steril 1996; Suppl.: S99.
Vanderzwalmen P, Zech H, Birkenfeld A et al. Intracytoplasmic injection of spermatids retrieved from testicular tissue: influence of testicular pathology, type of selected spermatids and oocyte activation. Hum Reprod 1997; 12: 1203–1213.
Antinori S, Versaci C, Dani G, Antinori M, Pozza D, Selman HA. Fertilization with human testicular spermatids: four successful pregnancies. Hum Reprod 1997; 12: 286–291.
Yamanaka K, Sofikitis NV, Miyagawa I et al. Ooplasmic round spermatid nuclear injection procedures as an experimental treatment for nonobstructive azoospermia. J Assist Reprod Genet 1997; 14: 55–62.
Amer M, Soliman E, el-Sadek M, Mendoza C, Tesarik J. Is complete spermiogenesis failure a good indication for spermatid conception? Lancet 1997; 350: 116.
Kahraman S, Polat G, Samli M et al. Multiple pregnancies obtained by testicular spermatid injection in combination with intracytoplasmic sperm injection. Hum Reprod 1998; 13: 104–110.
Barak Y, Kogosowski A, Goldman S, Soffer Y, Gonen Y, Tesarik J. Pregnancy and birth after transfer of embryos that developed from single-nucleated zygotes obtained by injection of round spermatids into oocytes. Fertil Steril 1998; 70: 67–70.
Al-Hasani S, Ludwig M, Palermo I et al. Intracytoplasmic injection of round and elongated spermatids from azoospermic patients: results and review. Hum Reprod 1999; 14: 97–107.
Ghazzawi IM, Alhasani S, Taher M, Souso S. Reproductive capacity of round spermatids compared with mature spermatozoa in a population of azoospermic men. Hum Reprod 1999; 14: 736–740.
Levran D, Nahum H, Farhi J, Weissman A. Poor outcome with round spermatid injection in azoospermic patients with maturation arrest. Fertil Steril 2000; 74: 443–449.
Vicdan K, Isik AZ, Delilbaşi L. Development of blastocyststage embryos after round spermatid injection in patients with complete spermiogenesis failure. J Assist Reprod Genet 2001; 18: 78–86.
Khalili MA, Aflatoonian A, Zavos PM. Intracytoplasmic injection using spermatids and subsequent pregnancies: round versus elongated spermatids. J Assist Reprod Genet 2002; 19: 84–86.
Sousa M, Cremades N, Silva J et al. Predictive value of testicular histology in secretory azoospermic subgroups and clinical outcome after microinjection of fresh and frozenthawed sperm and spermatids. Hum Reprod 2002; 17: 1800–1810.
Saremi A, Esfandiari N, Salehi N, Saremi MR. The first successful pregnancy following injection of testicular round spermatid in Iran. Arch Androl 2002; 48: 315–319.
Amarin ZO, Jamal HS, Rouzi AA. Successful pregnancy after round spermatid microinjection. Saudi Med J 2002; 23: 113–114.
Ulug U, Bener F, Akman MA, Bahceci M. Partners of men with Klinefelter syndrome can benefit from assisted reproductive technologies. Fertil Steril 2003; 80: 903–906.
Benkhalifa M, Kahraman S, Biricik A et al. Cytogenetic abnormalities and the failure of development after round spermatid injections. Fertil Steril 2004; 81: 1283–1288.
Yanagida K, Yazawa H, Katayose H. Oocyte activation induced by spermatids and the spermatozoa. Int J Androl 2000; 23: 63–65.
Winston NJ, Braude PR, Johnson MH. Are failed-fertilized human oocytes useful? Hum Reprod 1993; 8: 503–507.
Sjögren A, Lundin K, Hamberger L. Intracytoplasmic sperm injection of 1 day old oocytes after fertilization failure. Hum Reprod 1995; 10: 974–975.
Chen C, Kattera S. Rescue ICSI of oocytes that failed to extrude the second polar body 6 h post-insemination in conventional IVF. Hum Reprod 2003; 18: 2118–2121.
Suganuma R, Walden CM, Butters TD et al. Alkylated imino sugars, reversible male infertility-inducing agents, do not affect the genetic integrity of male mouse germ cells during short-term treatment despite induction of sperm deformities. Biol Reprod 2005; 72: 805–813.
DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet 2003; 72: 156–160.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yanagida, K., Fujikura, Y. & Katayose, H. The present status of artificial oocyte activation in assisted reproductive technology. Reprod Med Biol 7, 133–142 (2008). https://doi.org/10.1111/j.1447-0578.2008.00210.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1111/j.1447-0578.2008.00210.x