Abstract
Aim
The present study was designed to investigate the effect of amino acids and their dipeptides in the medium related to the urea cycle on the motility, viability, acrosome reaction (AR) and accumulation of ammonia in the medium over different incubation periods in porcine spermatozoa and to assess the utilization of glucose.
Methods
Porcine spermatozoa were washed, swim-up and incubated at 37°C for 0–4 h in mTALP medium supplemented with 75–600 μmol/L ammonia. Amino acids (1.0 mmol) or their dipeptides (2.0 mmol) were added individually to the mTALP medium containing either no ammonia or 300 μmol/L of ammonia. The viability and AR of porcine spermatozoa were assessed using the triple-staining technique and the accumulation of ammonia in the medium was measured using the indophenol method.
Results
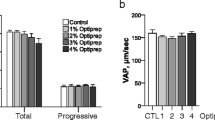
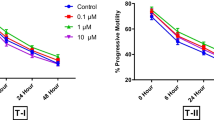
The motility viability and AR were adversely affected (P < 0.05) by concentrations of ammonia ≥300 μmol/L compared with the control. Supplementation of L-alanyl-L-glutamine (AlaGln), L-glycyl-L-glutamine (GlyGln) and AlaGln + GlyGln in the presence of 300 μmol/L ammonia significantly increase (P < 0.05) the rate of motility, viability, AR, incorporation, accumulation of ammonia and oxidation of 14C(U)-glucose compared with the ammonia supplement control.
Conclusion
AlaGln and GlyGln in mTALP medium were more stable and effective than the individual amino acids in reducing the accumulation of ammonia, and subsequently increasing the rate of AR and the utilization of glucose in porcine spermatozoa.
Similar content being viewed by others
References
Ka HH, Sawai K, Wang WH, Im KS, Niwa K. Amino acids in maturation medium and presence of cumulus cells at fertilization promote male pronuclear formation in porcine oocytes matured and penetrated in vitro. Biol Reprod 1997; 57: 1483–1487
Gassner FX, Hopwood ML. Seminal amino acid and carbohydrate pattern of bulls with normal and abnormal testes function. Proc Soc Exp Biol Medical 1952; 81: 37–43.
Prior RL, Visek WJ. Effects of urea hydrolysis on tissue metabolite concentrations in rats. Am J Physiol 1972; 223: 1143–1149.
Visek WJ, Kolodny GM, Gross PR. Ammonia effects in cultures of normal transformed 3T3 cells. J Cell Physiol 1972; 80: 373–381.
Gardner DK, Lane M. Amino acids and ammonium regulate mouse embryo development in culture. Biol Reprod 1993; 48: 377–385.
Lane M, Gardner DK. Ammonium induces aberrant blastocyst differentiation, metabolism, pH regulation, gene expression and subsequently alters fetal development in the mouse. Biol Reprod 2003; 69: 1109–1117.
Kim SC, Kim HW. Effects of nitrogenous components of urine on sperm motility: an in vitro study. Int J Androl 1998; 21: 29–33.
Eagle H. Utilization of dipeptides by mammalian cells in tissue culture. Proc Soc Exp Biol Med 1955; 89: 96–99.
Adibi SA, Lochs H, Abumrad NN, Daniel H, Vazquez JA. Removal of glycyl glutamine by individual tissues: mechanism and impact on amino acid fluxes in postabsorption and starvation. J Nutr 1993; 123: 325–331.
Roth E, Ollenschlager G, Hamilton G et al. Influence of two glutamine-containing dipeptides on growth of mammalian cells. In Vitro Cell. Dev Biol 1988; 24: 696–698.
Biggers JD, McGinnis LK, Lawitts JA. Enhanced effect of glycyl-L-glutamine on mouse preimplantation embryos in vitro. Reprod Biomed Online 2004; 9: 59–69.
Tareq KMA, Miah AG, Salma U, Yoshida M, Tsujii H. Effect of amino acids and dipeptides on accumulation of ammonia in the medium during in vitro maturation and fertilization of porcine oocytes. Rep Med Biol 2007; 6: 165–170.
Hossain MS, Hyeong LJ, Miah AG, Tsujii H. Effect of fatty acids bound to bovine serum albumin-V on acrosome reaction and utilization of glucose in boar spermatozoa. Reprod Med Biol 2007; 6: 109–115.
Miah AG, Tareq KMA, Hamano K, Kohsaka T, Tsujii H. Effect of relaxin on acrosome reaction and utilization of glucose in boar spermatozoa. J Reprod Dev 2006; 52: 773–779.
Hossain MS, Tareq KMA, Hamano K, Tsujii H. Effect of fatty acid on boar sperm motility, viability and acrosome reaction. Reprod Med Biol 2007; 6: 235–239.
Parrish JJ, Krogenaes A, Susko-Parrish JL. Effect of bovine sperm separation by either swim-up or Percoll method on success of in vitro fertilization and early embryonic development. Theriogenology 1995; 15: 859–869.
Flaherty CO, Rodriguez P, Srivastava S. L-Arginine promotes capacitation and acrosome reaction in cryopreseved bovine spermatozoa. Bio Biop Act 2004; 1674: 215–221.
Vandevoort CA, Overstreet JW. Effect of glucose and other energy substrates on the hyperactivated motility of macaque sperm and the zona pellucida induced acrosome reaction. J Androl 1995; 16: 327–333.
Johnson LA, Aalbers JG, Grooten HJG. Artificial insemination of swine: fecundity of boar semen stored in Beltsville TS (BTS), modified Modena (MM), or MR-A and inseminated on one, three and four days after collection. Zuchthyg 1988; 23: 49–55.
Parrish JJ, Susko-Parrish J, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod 1988; 38: 1171–1180.
Ooba T, Sricharoen P, Areekijsree M, Kitiyanat Y, Pavasuthipaisit K. Evaluation of acrosome reaction in bovine sperm by a triple staining technique. J Physiol Sci 1990; 3: 91–104.
Tareq KMA, Obata R, Miah AG, Hamano K, Tsujii H. Ammonia concentration in porcine ovarian developing follicles. J Mamm Ova Res 2005; 22: 185–189.
Llanos MN, Ronco AM, Aguirre MC, Meizel S. Hamster sperm glycine receptor: evidence for its presence and involvement in the acrosome reaction. Mol Reprod Dev 2001; 58: 205–215.
Melendrez CS, Meizel S. Studies of porcine and human sperm suggesting a role for a sperm glycine receptor/Cl-channel in the zona pellucida-initiated acrosome reaction. Biol Reprod 1995; 53: 676–683.
Tosic J, Walton A. Metabolism of spermatozoa. The formation and elimination of hydrogen peroxide by spermatozoa and effects on motility and survival. Biochem J 1950; 47: 199–212.
Brand K, Fekl W, Hintzenstern J, Langer K, Luppa P, Schroener C. Glutamine metabolism in lymphocytes of the rat. Metabolism 1989; 38: 29.
Christie A, Butler M. Glutamine-based dipeptides are utilized in mammalian cell culture by extracellular hydrolysis catalyzed by a specific peptidase. J Biotechnol 1994; 37: 277–290.
Hultin T. Incorporation of N15-labeled glycine and alanine into the proteins of developing sea urchin eggs. Exp Cell Res 1952; 3: 494.
Brinster RL. Uptake and incorporation of amino acids by the preimplantation mouse embryo. J Reprod Fert 1971; 27: 329–338.
Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod 2004; 71: 540–547.
Tsujii H, Ohta E, Miah AG, Hossain MS, Slma U. Effect of fructose on motility, acrosome reaction and in vitro fertilization capability of boar spermatozoa. Reprod Med Biol 2006; 5: 255–261.
Vandevoort CA, Overstreet JW. Effects of glucose and other energy substrates on the hyperactivated motility of macaque sperm and the zona pellucida-induced acrosome reaction. J Androl 1995; 16: 327–333.
Wongsrikeao P, Otoi T, Taniguchi M et al. Effects of hexoses on in vitro oocyte maturation and embryo development in pigs. Theriogenology 2006; 65: 332–343.
Garbers DL, Kopf GS. The regulation of spermatozoa by calcium cyclic nucleotides. Adv Cyclic Nucleotide Res 1980; 13: 251–306.
Bartsch O, Bartlick B, Ivell R. Relaxin signalling links tyrosine phosphorylation to phosphodiesterase and adenylyl cyclase activity. Mol Hum Reprod 2001; 7: 799–809.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tareq, K.M.A., Hossain, M.S., Akter, Q.S. et al. Effect of amino acids and dipeptides on the acrosome reaction and accumulation of ammonia in porcine spermatozoa. Reprod Med Biol 7, 123–131 (2008). https://doi.org/10.1111/j.1447-0578.2008.00209.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1111/j.1447-0578.2008.00209.x