Abstract
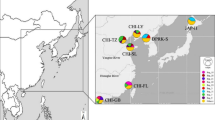
Allozyme variation of the littleneck clam Ruditapes philippinarum was evaluated in four samples from Nameishi and Matsuo in the Ariake Sea, Ryugatake and Ushibuka in the Shiranui Sea off Kyushu Island, Japan, and in one sample from Jinzhou, China, in the Bohai Sea. A Ruditapes bruguieri sample imported from the Korean Bay off Nampo, North Korea was also studied. Among the R. philippinarum samples, heterozygosity varied from 0.265 to 0.301 and F is estimates indicated significant homozygosity excess in 15 of 40 loci analyzed. Deviations from Hardy-Weinberg equilibrium were significant in all samples (P<0.05). Pairwise F ST estimates indicate that genetic differences between the Chinese and Japanese samples were very low, but significantly different from zero. Mixture proportions with 95% confidence intervals of Chinese R. philippinarum in Nameishi and Matsuo were estimated at 0.4098 [0.2512, 0.5705] and 0.4899 [0.3262, 0.6540], respectively. However, genetic invasion of stocked Chinese R. philippinarum into wild populations in the Ariake Sea remains uncertain due to the low precision of the estimates caused by the high similarity of allele frequencies between Jinzhou and the Ariake Sea.
Similar content being viewed by others
References
Ministry of Agriculture Forestry and Fisheries. Annual Statistics of Fisheries and Aquaculture Production in 1964–2004. Association of Agriculture and Forestry Statistics, Tokyo 1966–2006 (in Japanese).
Sekiguchi H, Ishii R. Drastic decreasing of annual catch yields of the manila clam Ruditapes philippinarum in Ariake Sound, Southern Japan. Oceanogr. Japan 2003; 12: 21–36 (in Japanese).
Toba M, Littleneck clam. In: Mori K (ed.). Aquaculture System, Vol. 3: Molluscs, Crustaceans, Sea Urchins and Algae. Koseisha Koseikaku. Tokyo. 2005; 287–198 (in Japanese).
World Conservation Union. Species survival commission in collaboration with the commission on ecology and the commission on environmental policy, law and administration. IUCN Position Statement of Translocation of Living Organisms. World Conservation Union. Gland. 1987.
Schwartz MK, Luikart G, Waples RS. Genetic monitoring as a promising tool for conservation and management. TRENDS Ecol. Evol. 2007; 22: 25–33.
Kijima A, Taniguchi N, Mori N, Hagiwara J. Genetic variability and breeding structure in Ruditapes philippinarum. Rep. Usa Mar. Biol. Inst., Kochi Univ. 1987; 9: 173–181 (in Japanese).
Oniwa K, Nakano M, Fujio Y. Heterogeneity within and between geographical populations of the short-necked clam, Ruditapes philippinarum. Tohoku J. Agric. Res. 1988; 38: 49–60.
Yokogawa K. Morphological variabilities and genetic features in Japanese common clam Ruditapes philippinarum. VENUS 1998; 57: 121–132 (in Japanese).
Sekine Y, Yamakawa H, Takazawa S, Lin Y, Toba M. Geographic variation of the COX1 gene of the short-neck clam Ruditapes philippinarum in coastal regions of Japan and China. VENUS 2006; 65: 229–240 (in Japanese).
Min DK. Mollusks in Korea (revised supplementary edition). Min Molluscan Research Institute, Seoul, 2004 (in Korean).
Fujio Y, Ikeda M. Isozyme and DNA analyses for population genetic research. In: Fujio Y (ed.). Study for the Conservation of Aquatic Organisms as Genetic Resources. Oyster Research Institute. Sendai. 1999; 13–41 (in Japanese).
Clayton JW, Tretiak DN. Amine-citrate buffers for pH control in starch gel electrophoresis. J. Fish. Res. Board Can. 1972; 29: 1169–1172.
Shaw CR, Prasad R. Starch gel electrophoresis of enzymes—a compilation of recipes. Biochem. Genet. 1970; 4: 297–320.
Raymond M, Rousset F. Genepop version 1.2: population genetics software for exact tests and ecumenicism. J. Hered. 1995; 86: 248–249.
Kitada S, Kitakado T, Kishino H. Empirical Bayes inference of pairwise F ST and its distribution in the genome. Genetics 2007; 177: 861–873.
Kumar S, Tamura K, Nei M. Mega 3:integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 2004; 5: 150–163.
Fournier D, Beacham TD, Riddell BE, Busack CA. Estimating stock composition in mixed-stock fisheries using morphometric, meristic and electrophoretic characteristics. Can. J. Fish. Aquat. Sci. 1984; 41: 400–408.
Millar RB. Maximum likelihood estimation of mixed stock fishery composition. Can. J. Fish. Aquat. Sci. 1987; 44: 583–590.
Kishino H, Kitada S, Hiramatsu K. Sampling scheme for the estimation of the stock composition in the mixed population based on genetic data. Nippon Suisan Gakkaishi 1994; 60: 359–364.
Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Chapman & Hall, New York, NY. 1993.
Hare MP, Karl SA, Avise JC. Anonymous nuclear DNA markers in the American oyster and their implications for the heterozygote deficiency phenomenon in marine bivalyes. Mol. Biol. Evol. 1996; 13: 334–345.
Mallet AL, Zouros E, Gartner-Kepkay KE, Freeman KR, Dickie LM. Larval viability and heterozygote deficiency in populations of marine bivalves: evidence from pairmatings of mussels. Mar. Biol. 1985; 87: 165–172.
Zouros E, Foltz DW. Possible explanations of heterozygote deficiency in bivalve molluses. Malacologia 1984; 25: 583–591.
Raymond M, Vaanto RL, Thomas F, Rousset F, De Meeus T, Renaud F. Heterozygote deficiency in the mussel Mytilus edulis species complex revisited. Mar. Ecol. Prog. Ser. 1997; 156: 225–237.
Plutchak LL, Simmons RE, Woodruff DS. Multilocus allozyme heterozygote deficiencies in Crepidula onyx: geographic and temporal patterns among adult snails in Mission Bay, California. J. Moll Stud. 2006; 72: 337–348.
Tracey ML, Bellet NF, Gravem CD. Excess allozyme homozygosity and breeding population structure in the mussel Mytilus californianus. Mar. Biol. 1975; 32: 303–311.
Fairbrother JE, Beaumont AR. Heterozygote deficiencies in a cohort of newly settled Mytilus edulis spat. J. Mar. Biol. Assoc. UK 1993; 73: 647–653.
Gaffney PM. Heterosis and heterozygote deficiencies in marine bivalves: more light? In: Beaumont AR (ed.) Genetics and Evolution of Aquatic Organisms. Chapman & Hall, London, 1994; 146–153.
The Oceanographic Society of Japan. Restoration of Ariake Sea’s Ecosystem. Koseisha-Koseikaku, Tokyo 2005 (in Japanese).
Sato M (ed.), Life in Ariake Sea: Biodiversity in Tidal Flats and Estuaries. Kaiyusha, Tokyo. 2000 (in Japanese).
Passamonti M, Boore JL, Scali V. Molecular evolution and recombination in gender-associated mitochondrial DNAs of the Manila clam Tapes philippinarum. Genetics 2003; 164: 603–611.
Yasuda N, Nagai S, Yamaguchi S, Lian CL, Hamaguchi M. Development of microsatellite markers for the manila clam Ruditapes philippinarum. Mol. Ecol. Notes 2007; 7: 43–45.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vargas, K., Asakura, Y., Ikeda, M. et al. Allozyme variation of littleneck clam Ruditapes philippinarum and genetic mixture analysis of foreign clams in Ariake Sea and Shiranui Sea off Kyushu Island, Japan. Fish Sci 74, 533–543 (2008). https://doi.org/10.1111/j.1444-2906.2008.01556.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1111/j.1444-2906.2008.01556.x