Abstract
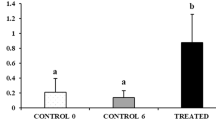
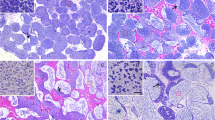
This study was undertaken to describe and quantitatively analyze the pattern of development of gametes in the Japanese scallop Patinopecten yessoensis. Incorporation of 5-bromo-2′-deoxyuridine (BrdU) into gonial cells was used to quantitatively detect mitotically active gonial cells. Oogonia increased in number from November to December and decreased rapidly to March. A small number of oocytes was detected in November. Oocytes steadily increased in number and size up to March. The number of spermatogonia slightly increased from November to December, and increased markedly from January to March. Both ratios of BrdU-immunopositive gonial cells in the ovary and testis to gonial cells moderately increased from September to December. The ratio of BrdU-immunopositive spermatogonia to gonial cells drastically increased from January to February and kept an elevated level in March, whereas the oogonia started to disappear in January. The results suggest that the pattern of proliferation of gonial cells can be divided into two phases: (i) phase I, oogonia and spermatogonia slowly proliferate through the growing stage; and (ii) phase II, oogonia develop into oocytes and spermatogonia begin to proliferate rapidly through the mature and spawning stages. The proliferation of gonial cells is likely under different endocrine controls in phases I and II.
Similar content being viewed by others
References
Osanai K. Seasonal gonad development and sex alteration in the scallop, Patinopecten yessoensis. Bull. Mar. Biol. St. Asamushi, Tohoku Univ. 1975; 15: 81–88.
Osada M, Takamura T, Sato H Mori K. Vitellogenin synthesis in the ovary of scallop, Patinopecten yessoensis: control by estradiol-17β and the central nervous system. J. Exp. Zool. A. Comp. Exp. Biol. 2003; 299: 172–179.
Li Q, Osada M, Suzuki T, Mori K. Change in vitellin during oogenesis and effect of estradiol-17β on vitellogenesis in the Pacific oyster Crassostrea gigas. Invertebr. Reprod. Dev. 1998; 33: 87–93.
Osada M, Harata M, Kishida M, Kijima A. Molecular cloning and expression analysis of vitellogenin in scallop. Patinopecten yessoensis (Bivalvia, Mollusca). Mol. Reprod. Dev. 2004; 67: 273–281.
Sastry AN. Pelecypoda (exclusive ostreidae). In: Giese AC, Pearse JS (eds). Reproduction of Marine Invertebrates, Vol. V. Academic Press, New York, NY. 1979; 113–292.
Awaji M, Suzuki T. The pattern of cell proliferation during pearl sac formation in the pearl oyster. Fish. Sci. 1995; 61: 747–751.
Miura T, Miura C. Japanese eel: a model for analysis of spermatogenesis. Zool. Sci. 2001; 18: 1055–1063.
Nakamura S, Osada M, Kijima A. Involvement of GnRH neuron in the spermatogonial proliferation of the scallop, Patinopecten yessoensis. Mol. Reprod. Dev. 2007; 74: 108–115.
Matsumoto T, Osada M, Osawa Y, Mori K. Gonadal estrogen profile and immunohistochemical localization of steroidogenic enzymes in the oyster and scallop during sexual maturation. Comp. Biochem. Physiol. 1997; 118B: 811–817.
Osada M, Tawarayama H, Mori K. Estrogen synthesis in relation to gonadal development of Japanese scallop, Patinopecten yessoensis: gonadal profile and immunolocalization of P450 aromatase and estrogen. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004; 139: 123–128.
Minuscci S, Di Matteo L, Pierantoni R, Varriale B, Rastogi RK, Chieffi G. In vitro and in vivo stimulatory effect of a Gonadotropin-releasing hormone analog (HOE 766) on spermatogonial multiplication in the frog, Rana esculenta. Endocrinology 1986; 119: 731–736.
Di Matteo L, Minucci S, Fasano S, Pierantoni P, Varriale B, Chieffi G. A gonadotropin-releasing hormone (GnRH) antagonist decreases androgen production and spermatogonial multiplication in frog (Rana esculenta). indirect evidence for the existence of GnRH or GnRH-like material receptors in the hypophysis and testis. Endocrinology 1988; 122: 62–67.
Minucci S, Di Matteo L, Chieffi P, Pierantoni R, Fasano S. 17β-estradiol effects on mast cell number and spermatogonial mitotic index in the testis of the frog, Rana esculenta. J. Exp. Zool. 1997; 278: 93–100.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Osada, M., Nakamura, S. & Kijima, A. Quantitative analysis of pattern of gonial proliferation during sexual maturation in Japanese scallop Patinopecten yessoensis . Fish Sci 73, 1318–1324 (2007). https://doi.org/10.1111/j.1444-2906.2007.01470.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1111/j.1444-2906.2007.01470.x