Abstract
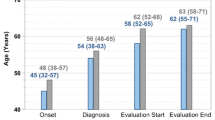
A postmarketing surveillance study was undertaken to investigate the safety and efficacy of interferon-α for human T-cell lymphotropic virus type 1 (HTLV-1)-associated myelopathy (HAM) under routine treatment conditions. A total of 273 cases from 91 medical institutions were registered into the survey. So far, 167 cases had been evaluated for safety and 152 for efficacy. The efficacy evaluation was rated based on clinical symptoms of HAM. Efficacy ratio (rate of patients assessed as “modest to markedly improved” and “mildly improved”) at 4 weeks was 66.2%. Factors that significantly affected efficacy ratio at 4 weeks was initial Osame’s motor disability score (OMDS) before interferon-α therapy and duration and stage of illness. Sustained improvement of OMDS for at least 5 months after stopping interferon-α was observed in 11 of 30 patients (36.7%). A total of 536 adverse drug reactions (ADRs) occurred in 146 patients, 46 of which were serious. Because some of these ADRs occurred late, it is necessary to watch out for them during long-term treatment.
Similar content being viewed by others
References
Izumo S, Goto I, Itoyama Y, Okajima T, Watanabe S, Kuroda Y, Araki S, Mori M, Nagataki S, Matsukura S, Akamine T, Nakagawa M, Yamamoto I, Osame M (1996). Interferonalpha is effective in HTLV-I-associated myelopathy: a multicenter, randomized, double-blind, controlled trial. Neurology 46: 1016–1021.
Matsuzaki T, Nakagawa M, Nagai M, Usuku K, Higuchi I, Arimura K, Kubota H, Izumo S, Akiba S, Osame M (2001). HTLV-I proviral load correlates with progression of motor disability in HAM/TSP: Analysis of 239 HAM/TSP patients including 64 patients followed up for 10 years. J NeuroVirol 7: 228–234.
Nagai M, Usuku K, Matsumoto W, Kodama D, Takenouchi N, Moritoyo T, Hashiguchi S, Ichinose M, Bangham CR, Izumo S, Osame M (1998). Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J NeuroVirol 4: 586–593.
Nakagawa M, Higashi K, Matsuzaki T, Saito M (2004). Prognosis of 314 patients with HAM and HTLV-I proviral load. Sogo Rinsho 53: 2103–2110.
Nakagawa M, Izumo S, Ijichi S, Kubota H, Arimura K, Kawabata M, Osame M (1995). HTLV-I-associated myelopathy: analysis of 213 patients based on clinical features and laboratory findings. J NeuroVirol 1: 50–61.
Nakagawa M, Nakahara K, Maruyama Y, Kawabata M, Higuchi I, Kubota H, Izumo S, Arimura K, Osame M (1996). Therapeutic trials in 200 patients with HTLV-I-associated myelopathy/tropical spastic paraparesis. J NeuroVirol 2: 345–355.
Osame M (1990). Review of WHO Kagoshima meeting and diagnostic guidelines for HAM/TSP. In: Human retrovirology: HTLV. Blattner WA (ed). New York: Raven Press, pp 191–197.
Osame M, Matsumoto M, Usuku K, Izumo S, Ijichi N, Amitani H, Tara M, Igata A (1987). Chronic progressive myelopathy associated with elevated antibodies to HTLV-I and adult T-cell leukemia-like cells. Ann Neurol 21: 117–122.
Osame M, McArthur JC (1992). Neurological manifestations of infection with human T cell lymphotropic virus type I. In: Diseases of the nervous system: clinical neurobiology, 2nd ed. vol 2. Asbury AK et al (eds). Philadelphia: WB Saunders, pp 1331–1339.
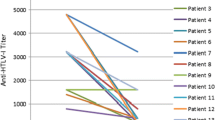
Saito M, Nakagawa M, Kaseda S, Matsuzaki T, Jonosono M, Eiraku N, Kubota R, Takenouchi N, Nagai M, Furukawa Y, Usuku K, Izumo S, Osame M (2004). Decreased human T lymphotropic virus type I (HTLV-I) provirus load and alteration in T cell phenotype after interferon-a therapy for HTLV-I—associated myelopathy/tropical spastic paraparesis. J Infect Dis 189: 29–40.
Thierry V, Jacques D (1994). Clinical toxicity of the interferons. Drug Safety 10: 115–150.
Yamasaki K, Kira J, Koyanagi Y, Kawano Y, Miyano-Kurosaki N, Nakamura M, Baba E, Suzuki J, Yamamoto A, Yamamoto N, Kobayashi T (1997). Long-term, high dose interferon-alpha treatment in HTLV-I-associated myelopathy/tropical spastic paraparesis: a combined clinical, virological and immunological study. J Neurol Sci 147: 135–144.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Dainippon Sumitomo Pharma Co., Ltd.
This work was supported in part by a Neuroimmunological Disease Research Committee grant from the Ministry of Health and Welfare, Japan.
Rights and permissions
About this article
Cite this article
Arimura, K., Nakagawa, M., Izumo, S. et al. Safety and efficacy of interferon-α in 167 patients with human T-cell lymphotropic virus type 1-associated myelopathy. Journal of NeuroVirology 13, 364–372 (2007). https://doi.org/10.1080/13550280701397627
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1080/13550280701397627