Abstract
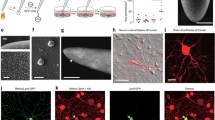
Semliki Forest virus (SFV) vectors are widely used in neurobiological studies because they efficiently infect neurons. As with any viral vector, they possess a limited cloning capacity, so infection with different SFV vectors may be required to introduce multiple transgenes into individual cells. However, this approach is limited by superinfection exclusion. The authors examined marker expression in baby hamster kidney cells, mouse cortical neurons, and rat hippocampal neurons using different fluorophore-encoding vectors that are based on the wild-type SFV4 strain and on the less cytopathic SFV4(PD) mutant, which carries two point mutations in nonstructural protein 2. For every fluorophore tested, SFV4(PD) gave higher (up to 22-fold) expression compared to SFV4. In infections using two and three different vectors, SFV4 caused relatively few multifluorescent baby hamster kidney cells when applied at 0-s, 15-min, or 2-h intervals. In contrast, SFV4(PD) permitted significantly enhanced marker coexpression, resulting in 46% doubly and 21% triply fluorescent baby hamster kidney cells, and 67% to 78% doubly fluorescent cortical and hippocampal neurons. At 15-min or 2-h addition intervals, SFV4(PD) still permitted 23% to 36% doubly fluorescent baby hamster kidney cells. The increased efficiency of SFV4(PD) in coexpressing separate markers from different viral particles suggests that mutations in nonstructural protein 2 affect alphaviral superinfection exclusion. The results demonstrate that SFV4(PD) is well-suited to coexpress multiple proteins in neuronal and non-neuronal cells. This capability is particularly valuable to express the various components of heteromeric protein complexes, especially when the individual cDNAs cannot be combined into single SFV particles.
Similar content being viewed by others
References
Agapov EV, Frolov I, Lindenbach BD, Pragai BM, Schlesinger S, Rice CM (1998). Non-cytopathogenic Sindbis RNA vectors for heterologous gene expression. Proc Natl Acad Sci U S A 95: 12989–12994.
Bausch SB, Patterson TA, Ehrengruber MU, Lester HA, Davidson N, Chavkin C (1995). Colocalization of mu opioid receptors with GIRK1 potassium channels in rat brain: an immunocytochemical study. Recept Channels 3: 221–241.
Bredenbeek PJ, Frolov I, Rice CM, Schlesinger S (1993). Sindbis virus expression vectors: packaging of RNA replicons by using defective helper RNAs. J Virol 67: 6439–6446.
Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, Tsien RY (2002). A monomeric red fluorescent protein. Proc Natl Acad Sci U S A 99: 7877–7882.
DeSouza S, Fu J, States BA, Ziff EB (2002). Differential palmitoylation directs the AMPA receptor-binding protein ABP to spines or to intracellular clusters. J Neurosci 22: 3493–3503.
Dryga SA, Dryga OA, Schlesinger S (1997). Identification of mutations in a Sindbis virus variant able to establish persistent infection in BHK cells: the importance of a mutation in the nsP2 gene. Virology 228: 74–83.
Ehrengruber MU (2002a). Alphaviral gene transfer in neurobiology. Brain Res Bull 59: 13–22.
Ehrengruber MU (2002b). Alphaviral vectors for gene transfer into neurons. Mol Neurobiol 26: 183–201.
Ehrengruber MU, Hennou S, Büeler H, Naim HY, Déglon N, Lundstrom K (2001). Gene transfer into neurons from hippocampal slices: comparison of recombinant Semliki Forest virus, adenovirus, adeno-associated virus, lentivirus, and measles virus. Mol Cell Neurosci 17: 855–871.
Ehrengruber MU, Lundstrom K (2002). Semliki Forest virus and Sindbis virus vectors. In: Current protocols in human genetics. Dracopoli NC, Haines JL, Korf BR, Morton CC, Seidman CE, Seidman JG, Smith DR (eds). New York: John Wiley & Sons, pp 12.2.1–12.2.23.
Ehrengruber MU, Lundstrom K, Schweitzer C, Heuss C, Schlesinger S, Gahwiler BH (1999). Recombinant Semliki Forest virus and Sindbis virus efficiently infect neurons in hippocampal slice cultures. Proc Natl Acad Sci U S A 96: 7041–7046.
Fazakerley JK, Boyd A, Mikkola ML, Kääriäinen L (2002). A single amino acid change in the nuclear localization sequence of the nsP2 protein affects the neurovirulence of Semliki Forest virus. J Virol 76: 392–396.
Frolov I, Agapov E, Hoffman TA, Pragai BM, Lippa M, Schlesinger S, Rice CM (1999). Selection of RNA replicons capable of persistent noncytopathic replication in mammalian cells. J Virol 73: 3854–3865.
Garmashova N, Gorchakov R, Frolova E, Frolov I (2006). Sindbis virus nonstructural protein nsP2 is cytotoxic and inhibits cellular transcription. J Virol 80: 5686–5696.
Graham A, Walker R, Baird B, Hahn CN, Fazakerley JK (2006). CNS gene therapy applications of the Semliki Forest virus 1 vector are limited by neurotoxicity. Mol Ther 13: 631–635.
Johnston RE, Wan K, Bose HR (1974). Homologous interference induced by Sindbis virus. J Virol 14: 1076–1082.
Kim KH, Rumenapf T, Strauss EG, Strauss JH (2004). Regulation of Semliki Forest virus RNA replication: a model for the control of alphavirus pathogenesis in invertebrate hosts. Virology 323: 153–163.
Li Z, Massengill JL, O’Dowd DK, Smith MA (1997). Agrin gene expression in mouse somatosensory cortical neurons during development in vivo and in cell culture. Neuroscience 79: 191–201.
Liang XH, Jiang HH, Levine B (1997). Expression of a biologically active antiviral antibody using a Sindbis virus vector system. Mol Immunol 34: 907–917.
Liljeström P, Garoff H (1991). A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Bio/Technology 9: 1356–1361.
Lundstrom K (2007). Alphaviruses: Semliki Forest virus and Sindbis virus as gene delivery vectors. In: Gene transfer: delivery and expression of DNA and RNA, a laboratory manual. Friedmann T, Rossi J (eds). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, pp 327–348.
Lundstrom K, Abenavoli A, Malgaroli A, Ehrengruber MU (2003). Novel Semliki Forest virus vectors with reduced cytotoxicity and temperature-sensitivity for long-term enhancement of transgene expression. Mol Ther 7: 202–209.
Maletic-Savatic M, Malinow R, Svoboda K (1999). Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283: 1923–1927.
Perri S, Driver DA, Gardner JP, Sherrill S, Belli BA, Dubensky TW Jr, Polo JM (2000). Replicon vectors derived from Sindbis virus and Semliki Forest virus that establish persistent replication in host cells. J Virol 74: 9802–9807.
Rikkonen M (1996). Functional significance of the nuclear-targeting and NTP-binding motifs of Semliki Forest virus nonstructural protein nsP2. Virology 218: 352–361.
Sawicki DL, Perri S, Polo JM, Sawicki SG (2006). Role for nsP2 proteins in the cessation of alphavirus minus-strand synthesis by host cells. J Virol 80: 360–371.
Scheer A, Björklöf K, Cotecchia S, Lundstrom K (1999). Expression of the α1 b-adrenergic receptor and G protein subunits in mammalian cell lines using the Semliki Forest virus expression system. J Recept Signal Transduct Res 19: 369–378.
Schlesinger S (2000). Alphavirus expression vectors. Adv Virus Res 55: 565–577.
Singh IR, Suomalainen M, Varadarajan S, Garoff H, Helenius A (1997). Multiple mechanisms for the inhibition of entry and uncoating of superinfecting Semliki Forest virus. Virology 231: 59–71.
Strauss JH, Strauss EG (1994). The alphaviruses: gene expression, replication, and evolution. Microbiol Rev 58: 491–562.
Takkinen K (1986). Complete nucleotide sequence of the nonstructural genes of Semliki Forest virus. Nucleic Acids Res 14: 5667–5682.
Tuittila MT, Santagati MG, Röyttä M, Määttä JA, Hinkkanen AE (2000). Replicase complex genes of Semliki Forest virus confer lethal neurovirulence. J Virol 74: 4579–4589.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to Drs. James H. and Ellen G. Strauss in vivid remembrance of their common ornithological excursions during Markus U Ehrengruber’s time at Caltech—with a look onto alphavirsues from the avian side.
This work was supported by grants from the NIH (NS48336), the McKnight Endowment Fund for Neuroscience, and the National Multiple Sclerosis Society.
Rights and permissions
About this article
Cite this article
Ehrengruber, M.U., Goldin, A.L. Semliki Forest virus vectors with mutations in the nonstructural protein 2 gene permit extended superinfection of neuronal and non-neuronal cells. Journal of NeuroVirology 13, 353–363 (2007). https://doi.org/10.1080/13550280701393204
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1080/13550280701393204