Summary
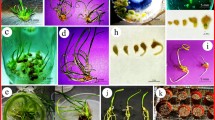
An efficient rapid and large-scale in vitro clonal propagation of the valuable medicinal herb Eclipta alba (Asteraceae) by enhanced axillary shoot proliferation in cotyledonary node segments was designed. The medium type, various carbon sources, plant growth regulators, and coconut water markedly influenced in vitro propagation of Eclipta alba. An in vitro plantlet production system has been investigated on Murashige and Skoog (MS) medium with the synergistic combination of benzyladenine (4.4μM), kinetin (4.6μM), 2-isopentenyladenine (4.9μM), gibberellic acid (1.4μM), 5% coconut water, and 3% sucrose which promoted the maximum number of shoots as well as beneficial shoot length: Subculturing of cotyledonary node segments on a similar medium enabled continuous production of healthy shoots with similar frequency. Rooting was highest (94.3%) on full strength. MS medium containing 9.8 μM indolebutyric acid. Micropropagated plants established in garden soil, farmyard soil, and sand (2∶1∶1) were uniform and identical to the donor plant with respect to growth characteristics as well as floral features. These plants grew normally without showing any morphological variation.
Similar content being viewed by others
References
Ajithkumar, D.; Seeni, S. Rapid clonal multiplication through in vitro axillary shoot proliferation of Aegle marmelos (L.) Corr., a medicinal tree. Plant Cell Rep. 17:422–426; 1998.
Anonymous. The wealth of India, raw materials, vol. 3. New Delhi: CSIR; 1952:127–128.
Bajaj, Y. P. S.; Reghunath, B. R.; Gopalakrishnan, P. K.; Elettaria cardamomum Maton (cardamom): aromatic compounds, in vitro culture studies and clonal propagation. In: Bajaj, Y. P. S., ed. Biotechnology in agriculture and forestry, vol. 21. Medicinal and aromatic plants. IV, Berlin: Springer-Verlag: 1993:132–147.
Borthakur, M.; Dutta, K.; Nath, S. C.; Sing, R. S. Micropropagation of Eclipta alba and Eupatorium adenophorum using a single-step nodal cutting technique. Plant Cell Tiss. Organ Cult. 62:239–242; 2000.
Brain, K. W.; Richard, A. C. Clonal propagation of pink ginger in vitro. Hort Science 28:1203; 1993.
Constantine, D. Round table conference, Gembloux. Belgium; 1978: 134.
De Paiva Neto, V. B.; Da Mota, T. R.; Otoni, W. C. Direct organogenesis from hypocotyl-derived explants of annatto (Bixa orellana). Plant Cell Tiss. Organ Cult. 75:159–167; 2003.
Franca, S. C.; Bertoni, B. W.; Pereira, A. M. S. Antihepatotoxic agent in micropropagated plantlets of Eclipta alba. Plant Cell Tiss. Organ Cult. 40:297–299; 1995.
Gamborg, O. L.; Miller, R. A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50:151–158; 1968.
Gao, S. L.; Zhu, D. N.; Cai, Z. H.; Jiang, Y.; Xu, D. R. Organ culture of a precious Chinese medicinal plant—Fritillaria unibracteata. Plant Cell Tiss. Organ Cult. 59:97–201; 1999.
Gawde, A. J.; Paratkar, G. T. Micropropagation of Eclipta alba—an approach to shorten the protocol. Indian J. Biotechnol. 3:128–132; 2004.
Gomez, K. A.; Gomez, A. A. Statistical procedures for agricultural research with emphasis on rice. Los Banos, Philippines: International Rice Research Institute; 1976.
Heide, O. M. Non-reversibility of gibberellin-induced inhibition of regulation in Begonia leaves. Physiol. Plant. 22:671–679; 1969.
Hisashi, H.; Yasuhiro, M. Micropropagation of Prunus mume. Plant Cell Tiss. Organ Cult. 46:265–267; 1996.
Hu, C. Y.; Wang, P. J. Meristem shoot tip and bud culture. In: Evans, D. A.; Sharp, W. R.; Ammirato, P. V.; Yamada, Y., eds. Handbook of plant cell culture. New York: Macmillan; 1983:177–227.
Indra, D. B.; Dhar, U. Micropropagation of Indian wild strawberry. Plant Cell Tiss. Organ Cult. 60:83–88; 2000.
Komalavalli, N.; Rao, M. V. In vitro micropropagation of Gymnema sylvestre—a multipurpose medicinal plant. Plant Cell Tiss. Organ Cult. 61:97–105; 2000.
Kozai, T. Micropropagation under photoautotrophic conditions. In: Debergh, P. C.; Zimmerman, R. H., eds. Micropropagation: technology and application. Dordrecht: Kluwer Academic Publishers; 1991:447–469.
Kukreja, A. K.; Mathur, A. K. Tissue culture studies in Duboisia myoporoides, I. Plant regeneration and clonal propagation by stem node cultures. Planta Med. 2:93–96; 1985.
Monsor, N.; Ismaila, D.; Yaye, K. G. D. In vitro multiplication of the semiarid forest tree. Balanites aegyptiaca (L) Del. African J. Biotechnol. 2:421–424; 2003.
Mors, W. B.; Nascimento, M. C.; Parente, J. P.; Silva, M. H.; Melo, P. A.; Suarez-Kurtz, G. Neutralization of lethal and myotoxic activities of South American rattlesnakes venom by extracts and constituents of the plant Eclipta prostata (Asteraceae). Toxicon 27:1003–1009; 1989.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497; 1962.
Nadganda, R. S.; Mascarenhas, A. P.; Madhusoodanan, K. Clonal multiplication of cardamom (Eletteria cardamomum Maton) by tissue culture. Plantation Crops 11:60–64; 1983.
Orning, L.; Hammarstrom, S.; Samuelsson, B., Leukotriene D: a slow reacting substance from rat basophilic leukemia cells. Proc. Natl Acad. Sci. USA 77:2014–2017; 1980.
Pattnaik, S. K.; Chand, P. K. In vitro propagation of the medicinal herbs Ocimum americanum L. and Ocimum sanctum L. Plant Cell Rep. 15:846–850; 1996.
Prakash, E.; Sha Valli Khan, P. S.; Sanam Reddy, P.; Rao, K. R. Regeneration of plants from seed-derived callus of Hybanthus enneaspermus L. Muell., a rare ethnobotanical herb. Plant Cell Rep. 18:873–878; 1999.
Pritchard, J.; Wyn-Jones, R. G.; Tomos, A. D. Turgor, growth and rheological gradients in wheat roots following osmotic stress. J. Exp. Bot. 42:1043–1049; 1991.
Pua, E. C.; Chong, C. Requirement for sorbitol (d-glucitol) as carbon source for in vitro propagation of Malus robusta No. 5. Can. J. Bot. 62:1545–1549; 1984.
Purohit, S. D.; Dave, A. Micropropagation of Sterculia urens Roxb.— an endangered tree species. Plant Cell Rep. 15:704–706; 1996.
Reghunath, B. R.; Bajaj, Y. P. S. Micropropagation of cardamom (Elettaria cardamomum Maton). In: Bajaj, Y. P. S., ed. Biotechnology in agriculture and forestry, vol. 19. High tech and micropropagation III. Berlin: Springer-Verlag; 1992:175–198.
Romano, A.; Noronha, C.; Martins-Loucao, M. A. Role of carbohydrates in micropropagation of Cork oak. Plant Cell Tiss. Organ Cult. 40:159–167; 1995.
Sahoo, Y.; Chand, P. K. Micropropagation of Vitex negundo L., a woody aromatic medicinal shrub, through high frequency axillary shoot proliferation. Plant Cell Rep. 18:301–307; 1998.
Sahoo, Y.; Pattnaik, S. K.; Chand, P. K. In vitro clonal propagation of medicinal plant Ocimum basilicum L. by axillary shoot proliferation. In Vitro Cell. Dev. Biol. Plant 33:293–296; 1997.
Sajina, A.; Mini, P. M.; John, C. Z.; Babu, K. N.; Ravindran, P. N.; Peter, K. V. Micropropagation of large cardamom (Amomum subulatum Roxb.). J. Spices Aromatic Crops 6:145–148; 1997.
Schenk, R. U.; Hildebrandt, A. C. Medium and technique for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 50:199–204; 1972.
Shamsudeen Varisai, M.; Jawahar, M.; Thiruvengadam, M.; Jeyakumar, M.; Jayabalan, N. Effect of cytokinins on the proliferation of multiple shoots in Horsegram (Macrotyloma uniflorum (Lam) Verde). J. Plant Biotechnol. 1:79–83; 1999.
Shirgurkar, M. V.; John, C. K.; Nadganda, R. S. Factors affecting invitro microrhizome production in turmeric. Plant Cell Tiss. Organ Cult. 64:5–11; 2003.
Shirin, F.; Kumar, S.; Mishra, Y. In vitro plantlet production system for Kaempferi galanga, a rare Indian medicinal herb. Plant Cell Tiss. Organ Cult. 63:193–197; 2000.
Sivakumar, G.; Krishnamurthy, K. V. Micropropagation of Gloriosa superba L.— an endangered species of Asia and Africa. Curr. Sci. 78:30–32; 2000.
Skirvin, R. M.; Chu, M. C.; Rukan, H. An improved medium for the in vitro rooting of Harbrite peach. Proc. III State Hort. Soc. 113:30–38; 1980.
Tejavathi, D. H.; Shailaja, K. S. Regeneration of plants from the cultures of Bacopa monnieri (L.) Pennell. Phytomorphology 49:447–452; 1999.
Thompson, M.; Thorpe, T. Metabolic and non-metabolic roles of carbohydrates. In: Bonga, J. M.; Durzan, D. J., eds. Cell and tissue culture in forestry. Dordrecht: Martinus Nijhoff; 1987:89–112.
Venkatachalam, P.; Jayabalan, N. P. Effect of auxins and cytokinins on efficient plant regeneration and multiple shoot formation from cotyledons and cotyledonary node explants of ground nut (Arachis hypogaea L.) by in vitro culture technology. Appl. Biochem. Biotechnol. 67:237–247; 1997.
Wagner, H.; Geyer, B.; Kiso, Y.; Rao, G. S. Coumestans as the main active principles of the liver drugs Eclipta alba and Wedelia calendulacea. Planta Med. 52:370–373; 1986.
Wondyifraw, T.; Surawit, W. Micropropagation of Krawan (Amomum krervanh Pierre ex Gagnep). Sci. Asia 30:9–150; 2004.
Zafar, R.; Sagar, B. P. S. In vitro plant regeneration of Eclipta alba and increased production of coumestans. Fitoterapia 70:348–356; 1999.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baskaran, P., Jayabalan, N. An efficient micropropagation system for Eclipta, alba—A valuable medicinal herb. In Vitro Cell.Dev.Biol.-Plant 41, 532–539 (2005). https://doi.org/10.1079/IVP2005667
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2005667