Summary
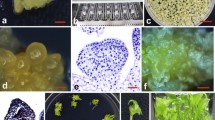
Despite intensive efforts, a reproducible and reliable method for transformation of sugarbeet plants is still lacking. Having examined several explants, we found that cells around the main vein of leaves of plantlets reared from tissue-cultured apical meristems are sufficiently competent for transformation and subsequent regeneration. A transformation protocol was designed by evaluating alterations in several parameters such as plant genotype, Agrobacterium strain, antibiotics, darkness and duration of co-culture period. An average transformation rate of 6.2% transformed shoots per explant was achieved as judged by Southern blotting. Consistent inactivation of reporter genes was correlated to multiple copies of transgenes present in some transformants. The necessary steps for rooting and planting of transformed shoots were also established.
Similar content being viewed by others
References
Connor-Ward, D.; Hinchee, A. W. M. Sugarbeet regeneration and transformation. Patent no. WO 0142480; 2001.
De Greef, W.; Jacobs, M. In vitro culture of the sugarbeet: description of a cell line with high regeneration capacity. Plant Sci. Lett. 17:55–61; 1979.
Feinberg, A. P.; Vogelstein, B. A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem. 132:6–13; 1983.
Gamborg, O. L.; Miller, R. A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50:151–158; 1968.
Hall, R. D.; Bruinsma, T. R.; Weyens, G. J.; Rosquin, I. J.; Denys, P. N.; Evans, I. J.; Lathouwers, J. E.; Lefebvre, M. P.; Dunwell, J. M.; Tunen, A. V.; Krens, F. A. A high efficiency technique for the generation of transgenic sugarbeets from stomatal guard cells. Nature Biotechnol. 14:1133–1138; 1996.
Horsch, R. B.; Fry, J. E.; Hoffmann, N.; Eicholz, D.; Rogers, S. G.; Fraley, R. T. A simple and general method for transferring genes into plants. Science 227:1229–1231; 1985.
Jacq, B.; Lesobre, O.; Sangwan, R. S.; Sangwan, B. S. Factors influencing T-DNA transfer in Agrobacterium-mediated transformation of sugarbeet. Plant Cell Rep. 12: 621–624; 1993.
Jefferson, R. A.; Kavanagh, T. A.; Bevan, M. W. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6:3901–3907; 1987.
Joersbo, M.; Donaldson, I.; Kreiberg, J.; Petersen, S. G.; Brunstedt J.; Okkels, F. T. Analysis of mannose selection used for transformation of sugarbeet. Mol. Breed. 4:111–117; 1998.
Joersbo, M.; Okkels, F. T. A novel principle of selection of transgenic plant cells: positive selection. Plant Cell Rep. 16:219–221; 1996.
Koncz, C.; Schell, J. The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol. Gen. Genet. 204:393–396; 1986.
Konward, B. K. Agrobacterium tumefaciens-mediated genetic transformation of sugarbeet (Beta vulgaris L.). J. Plant Biochem. Biotechnol. 3:37–41; 1994.
Krens, F. A.; Trifonova, A.; Keizer, L. C. P.; Hall, R. D. The effect of exogenously-applied phytohormones on gene transfer efficiency in sugarbeet (Beta vulgaris L.). Plant Sci. 116:97–106; 1996.
Lindsey, K.; Gallois, P. Transformation of sugarbeet (Beta vulgaris L.) by Agrobacterium tumefaciens. J. Exp. Bot. 41:529–536; 1990.
Mannerlöf, M.; Lennerfors, B. L.; Tenning, P. Reduced titer of BNYVV in transgenic sugarbeets expressing the BNYVV coat protein. Euphytica 90:293–299; 1996.
Mannerlöf, M.; Tuvesson, S.; Steem, P.; Tenning, P. Transgenic sugarbeet tolerant to glyphosate. Euphytica 94:83–91; 1997.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497; 1962.
Norouzi, P.; Yazdi-Samadi, B.; Malboobi, M. A. Investigating the effect of plant hormones on direct shoot regeneration from sugarbeet explants. Iranian J. Agric. Sci. 33:233–239; 2002.
Saghai-Maroof, M. A.; Soliman, K. M.; Jorgensen, R. A.; Allard, R. W. Ribosomal DNA spacer-length polymorphism in barley: Mendelian inheritance, chromosomal, location and population dynamics. Proc. Natl Acad. Sci. USA 81:8014–8018; 1984.
Sambrook, J.; Fritsch, E. F.; Maniatis, T. Molecular cloning: a laboratory manual, 2nd edn, vol. 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989.
Saunders, J. W.; Doley, W. P.; Theurer, J. C.; Yu, M. H. Somaclonal variation in sugarbeet. In: Bajaj, Y. P. S., ed. Biotechnology in agriculture and forestry, vol. 11. Heidelberg: Springer-Verlag Press; 1990:465–490.
Snyder, G. W.; Ingersoll, J. C.; Simigocki, A. C. Introduction of pathogen defense genes and a cytokinin biosynthesis gene into sugarbeet (Beta vulgaris L.) by Agrobacterium or particle bombardment. Plant Cell Rep. 18:829–834; 1999.
Velten, J.; Schell, J. Selection-expression plasmid vectors for use in genetic transformation of higher plants. Nucleic Acids Res. 13:6981–6998; 1985.
Winner, C. History of the crop. In: Cooke, D. A.; Scott, R. K., eds. The sugarbeet crop, London: Chapman and Hall 1993;1–36.
Wozniak, C. A. Transgenic sugarbeet: progress and development. In: Chopra, V. L.; Malik, V. S.; Bhat, S. R., eds. Applied plant biotechnology, Enfield: Science Publisher, Inc; 1999:301–324.
Wozniak, C. A.; Owens, L. D. Native β-glucuronidase activity in sugarbeet (Beta vulgaris L.). Physiol. Plant. 90:763–771; 1994.
Zhang, C. L.; Chen, D. F.; McCormac, A. C.; Scott, N. W.; Elliott, M. C.; Slater, A. Use of the GFP reporter as a vital marker for Agrobacterium-mediated transformation of sugarbeet (Beta vulgaris L.). Mol. Biotechnol. 17:109–117; 2001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Norouzi, P., Malboobi, M.A., Zamani, K. et al. Using a competent tissue for efficient transformation of sugarbeet (Beta vulgaris L.). In Vitro Cell.Dev.Biol.-Plant 41, 11–16 (2005). https://doi.org/10.1079/IVP2004589
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2004589