Summary
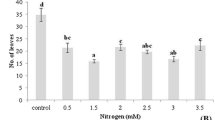
Although pineapple plants have been found to produce proteases ex vitro, most of the biotechnological investigations of this crop have been focused on propagation. The procedure involving the use of temporary immersion bioreactors is one of the most outstanding because of its high multiplication rate. We previously recorded specific protease activity in the culture medium during the pre-elongation step of this protocol. Therefore, we decided to modify the culture medium composition of this phase looking for an increase in protease excretion. Four independent experiments were performed to evaluate the effects of different levels of sucrose (0–350.4 mM), inorganic salts [0–200% Murashige and Skoog (MS) salt strength], inositol (0–2.20 mM), and thiamine (0–1.2μM). The following indicators were recorded: shoot fresh mass per bioreactor; and protein concentration, proteolytic activity, and specific protease activity in culture media. Specific protease activity, the most important indicator recorded, was highest with 262.8 mM sucrose, 100% MS salt strength, 0.3 μM thiamine and no inositol. Results shown here demonstrate that conditions adequate for propagation purposes (87.6 mM sucrose, 100% MS salt strength, 0.55 mM inositol, 0.3 μM thiamine) are not always adequate for protease excretion.
Similar content being viewed by others
References
Anson, M. L. The estimation of pepsin, trypsin, papain and cathepsin with hemoglobin. J. Gen. Physiol. 22:79; 1938.
Apte, P.; Kaklij, G.; Heble, M. Proteolytic enzymes bromelains in tissue cultures of Ananas sativus pineapple. Plant Sci. Lett. 14:52–62; 1979.
Avilés, X.; Guasch, A.; Vendrell, J. Activation of protein precursors. Res. Bull. 100(210):74–81; 1994 (in Spanish).
Bailey, A.; Light, N. Connective tissue In: Bayley, A., ed. Meat and meat products. London: Elsevier Science Publishers Ltd.; 1989:213–214.
Balla, T. Phosphatidylinositol 4-kinases. Biochem. Biophys. Acta 1436:69–85; 1998.
Barwale, U. B.; Kerns, H. R.; Wildholm, J. M. Plant regeneration from callus cultures of several soybean genotypes via embryogenesis and organogenesis. Planta 167:473–481; 1986.
Batkin, S.; Taussig, S.; Szekerezes, R. Modulation of pulmonary metastases (Lewis lung carcinoma) by bromelain, an extract of the pineapple stem (Ananas comosus). Cancer Inv. 6:241–242; 1988.
Beers, E. P.; Woffenden, B. J.; Zhao, C. Plant proteolytic enzymes: possible roles during programmed cell death. Plant Mol. Biol. 44:399–415; 2000.
Chávez, M.; Díaz, J.; Delfin, J.; Pérez, U. Topics of enzymology, vol. II, 1st edn. Havana: ENPES; 1990: 25–30, 41–48, 250–261 (in Spanish).
Cournac, L.; Dimon, B.; Carrier, P.; Lohou, A.; Chagvardieff, P. Growth and photosynthetic characteristics of Solanum tuberosum plantlets cultivated in vitro in different conditions of aeration, sucrose supply and CO2 enrichment. Plant Physiol. 97:112–117; 1991.
Daqyinta, M.; Benegas, R. Brief review of tissue culture of pineapple. Pineapple Newsl. 3:7–9; 1997.
Debergh, P. C.; Zimmerman, R. H. Micropropagation, technology and application. In: Debergh, P. C.; Zimmerman, R. H., eds. Micropropagation. Dordrecht: Kluwer Academic Publishers; 1991:45–69.
Desjardins, Y.; Hdider, C.; De Riek, J. Carbon nutrition, in vitro regulation and manipulation of carbon assimilation in micropropagated systems. In: Aitken Christie, J.; Kozai, T.; Smith, M. A. L., eds. Automation and environmental control in plant tissue culture. Dordrecht: Kluwer Academic Publishers; 1996:441–471.
Do, C. B.; Cormier, F. Effects of low nitrate and high sugar concentrations on anthocyanin content and composition of grape (Vitis vinifera L.) cell suspension. Plant Cell Rep. 9:500–504; 1991.
Drøbak, B. K.; Watkins, P. A. C. Inositol (1,4,5) trisphosphate production in plant cells: an early response to salinity and hyperosmotic stress. FEBS Lett. 481:240–244; 2000.
Engwerda, C. R.; Andrew, D.; Ladhams, A.; Mynott, T. L. Bromelain modulates T and B cell immune responses in vitro and in vivo. Cell. Immunol. 210:66–75; 2001.
Escalona, M.; Lorenzo, J. C.; González, B.; Daquinta, M.; Borroto, C.; González, J. L.; Desjardins, Y. Pineapple micropropagation in temporary immersion systems. Plant Cell Rep. 18:743–748; 1999.
Fang, Y.; Smith, M. A. L.; Pépin, M. F. Benzyladenine restores anthocyanin pigmentation in suspension cultures of wild Vaccinium pahalae. Plant Cell Tiss. Organ Cult. 54:113–122; 1998.
Flórez, J. Farmacolgía humana, vol. 76. 2nd edn. Madrid: Salvat; 1995:142–146.
Gao, W. Y.; Fan, L.; Paek, K. Y. Yellow and red pigment production by cell cultures of Carthamus tinctorius in bioreactor. Plant Cell Tiss. Organ Cult. 60:95–100; 2000.
George, E. F. Plant propagation by tissue culture, 2nd edn. London: Exegetics Ltd. 1993:524 pp.
Hanagata, N.; Ito, A.; Fukuju, Y.; Murata, K. Red pigment formation in cultured cells of Carthamus tinctorius L. Biosci. Biotechnol. Biochem. 6:44–47; 1992.
Hanagata, N.; Ito, A.: Uehara, H. Behavior of cell aggregate of Carthamus tinctorius L. cultured cells and correlation with red pigment formation. J. Biotechnol. 30:259–269; 1993.
Headon, D.; Walsh, G. The industrial production of enzymes. Biotechnol. Adv. 12:635–646; 1994.
Hernández, M.; Carvajal, C.; Santos, R.; Márquez, M.; Blanco, M.; González, J.; Chávez, M. Purification alternatives of obtained bromelain from different sources. Pineapple Newsl. 6:5; 1999.
Igbavboa, U.; Sieweke, H. J.; Leistner, E.; Röwer, I.; Hüsemann, W.; Barz, W. Alternative formation of anthraquinones and lipoquinones in heterotropic and photoautotrophic cell suspension cultures of Morinda lucida Benth. Planta 166:537–544; 1985.
Ikeda, M.; Ojima, K.; Ohira, K. Habituation in suspension-cultured soybean cells to thiamine and its precursors. Plant Cell Physiol. 20:733–740; 1979.
Kelly, G. S. Bromelain: a literature review and discussion of its therapeutic applications. Altern. Med. Rev. 1:405–410; 1996.
Kleef, R.; Delohery, T.; Boubjerg, D. Selective modulation of cell adhesion molecules on lymphocytes by bromelain protease 5. Pathobiology 64:339–346; 1996.
Kotvun, T.; Daie, J. End-product control of carbon metabolism in culturegrown sugar beet plants. Plant Physiol. 108:1647–1656; 1995.
La Valle, J.; Krinsky, D.; Hawkins, E. Natural therapeutics pocket guide. Hudson, OH: Lexi-Comp; 2000.
Lawrie, R. Meat science. London: Pergamon Press; 1985:195–197.
Leipner, J.; Ilen, F.; Saller, R. Therapy with proteolytic enzymes in rheumatic disorders. BioDrugs 15:779–789; 2001.
Loewus, F. A.; Murthy, P. N. Myo-inositol metabolism in plants. Plant Sci. 150:1–19; 2000.
Losada, E. Bromelain. In: Importancia de las enzimas en el asma ocupacional. http://www.alergoaragon.org/1999/tercera2.html; 1999 (accessed August 2001).
Lotti, T. Controlled clinical studies of bromeline in the treatment of urogenital inflammation. Drugs 46:144–146; 1993.
Lowry, O.; Rosebrough, N.; Farr, A.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275; 1951.
Massot, B.; Milesi, S.; Gontier, E.; Bourgaud, F.; Guckert, A. Optimized culture conditions for production of furanocoumarins by micropropagated shoots of Ruta graveolens. Plant Cell Tiss. Organ Cult. 62:11–19; 2000.
McArdle, A. False vitamins and vitaminoids. http://www.portalfitness.com/nutrition/vitamine/vitaminoides/htm; 2003 (accessed May 2003) (in Spanish).
McBrige, 1999. Bromelain. In: Bromelain—health food for bossy, too (anti-inflammatory). http://www.findarticles.com/1999/tercera2.html; November 1999 (accessed August 2001).
Melis, G. Clinical experience with metoxybutropate vs. bromelain in the treatment of female pelvic inflammation. Minerva Ginecol. 42:309–312; 1990.
Metzig, C.; Crabowska, E.; Eckert, K.; Rehse, K.; Maurer, H. Bromelain proteases reduce human platelet aggregation in vitro, adhesion to bovine endothelial cells and thrombus formation in rat vessels in vivo. In Vivo 13:7–12; 1999.
Meurs, C.; Basra, A. S.; Karssen, C. M.; van Loon, L. C. Role of abscisic acid in the induction of desiccation tolerance in developing seeds of Arabidopsis thaliana. Plant Physiol. 98:1484–1493; 1992.
Miernyk, J. A.; Rapp, B. J.; David, R.; Randall, D. D. Higher plant mitochondrial pyruvate dehydrogenase complexes. In: Moore, A. L.; Beechey, J., eds. Plant mitochondria. New York: Plenum Press; 1987:189–197.
Miller, A. Improved sausage casing. US patent 3 666 844; 1982.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497; 1962.
Peña, H. A.; Díaz, J. A.; Martínez, T. R. Tropical plant culture, vol. 1. 1st edn. Havana: ICFES; 1998:234 pp (in Spanish).
Pérez, A.; Nápoles, L.; Lorenzo, J. C.; Hernández, M. Protease excretion during pineapple micropropagation in temporary immersion bioreactors. In Vitro Cell. Dev. Biol. Plant 39:311–315; 2003.
Pierik, R. L. M. In vitro culture of higher plants, 1st edn. Madrid: Ediciones Mundi-Prensa; 1990;325 pp. (in Spanish).
Pospísilová, J.; Catsky, J.; Sesták, Z. Photosynthesis in plants cultivated in vitro. In: Pessaraki, M., ed. New York: Marcel Dekker; 1997:525–540.
Ransberger, K.; Stauder, G. Process of using catabolic enzymes for induction of tumor necrosis factor (TNF). US patent 5223406; 1993.
Salisbury, F. B.; Ross, C. W. Plant physiology, 4th edn. Belmont, CA: Wadsworth Publishing; 1992:127–148.
Sato, K.; Yamazaki, T.; Okuyama, E.; Yoshihira, K.; Shimomura, K. Anthraquinones production by transformed root cultures of Rubia tinctorium: influence of phytohormones and sucrose concentration. Phytochemistry 30:1507–1509; 1991.
Sepehr, F.; Ghorbanli, M. Effects of nutritional factors on the formation of anthraquinones in callus cultures of Rheum ribes. Plant Cell Tiss. Organ Cult. 68:171–175; 2002.
Stevenson, J. M.; Perera, I. Y.; Heilmann, I.; Persson, S.; Boss, W. F. Inositol signaling and plant growth. Trends Plant Sci. 5:252–258; 2000.
Targoni, O.; Tary, L.; Lehmann, P. Prevention of murine EAE by oral hydrolytic enzyme treatment. J. Autoimmun. 12:191–198; 1999.
Tichá, I.; Cáp, F.; Pacovská, D.; Hofman, P.; Haisel, D.; Capková, V.; Schäfer, C. Culture on sugar medium enhances photosynthetic capacity and light resistance of plantlets grown in vitro. Physiol. Plant. 102:155–162; 1998.
Trejo-Tapia, G.; Arias-Castro, C.; Rodríguez-Mendiola, M. Influence of the culture medium constituents and inoculum size on the accumulation of blue pigment and cell growth of Lavandula spica. Plant Cell Tiss. Organ Cult. 72:7–12; 2003.
Verma, P. C.; Singh, D.; Rahman, L. U.; Gupta, M. M.; Banerjee, S.: In vitro-studies in Plumbago zeylanica: rapid micropropagation and establishment of higher plumbagin yielding hairy root cultures. J. Plant Physiol. 159:547–552; 2002.
Xu, J. F.; Ying, P. Q.; Han, A. M.; Su, Z. G. Enhanced salidroside production in liquid-cultivated compact callus aggregates of Rhodiola sachalinensis: manipulation of plant growth regulators and sucrose. Plant Cell Tiss. Organ Cult. 55:53–58; 1998.
Zeevaart, J. A.; Creelmann, R. A. Metabolism and physiology of abscisic acid. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39:439–473; 1988.
Zenk, M. H.; Shulte, U.; El-Shagi, H. Regulation of anthraquinone formation by phenoxyacetic acids in Morinda cell cultures. Naturwissenschaften 71:266; 1984.
Zhang, Y. H.; Wang, H. Q.; Liu, S.; Yu, J. T.; Zhong, J. J. Regulation of apparent viscosity and O2 transfer coefficient by osmotic pressure in cell suspensions of Panax notoginseng. Biotechnol. Lett. 19:943–945; 1997.
Zhu, J. K. Plant salt tolerance. Trends Plant Sci. 6:66–71; 2001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérez, A., Nápoles, L., Carvajal, C. et al. Effect of sucrose, inorganic salts, inositol, and thiamine on protease excretion during pineapple culture in temporary immersion bioreactors. In Vitro Cell.Dev.Biol.-Plant 40, 311–316 (2004). https://doi.org/10.1079/IVP2004529
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2004529