Summary
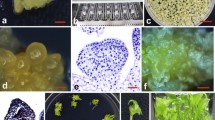
Hemp (Cannabis sativa L.) is cultivated in many parts of the world for ils fiber, oil, and seed. The development of new hemp cultivars with improved traits could be facilitated through the application of biotechnological strategies. The purpose of this study was to investigate the propagation of hemp in tissue culture and to establish a protocol for Agrobacterium-mediated transformation for foreign gene introduction. Stem and leaf segments from seedlings of four hemp varieties were placed on Murashige and Skoog medium with Gamborg B5 vitamins (MB) supplemented with 5 μM 2,4-dichlorophenoxyacetic acid (2,4-D) and 1 μM kinetin, 3% sucrose, and 8 gl−1 agar. Large masses of callus were produced within 4 wk for all cultivars. Suspension cultures were established in MB medium containing 2.5 μM 2,4-D. To promote embryogenesis or organogenesis, explants, callus, and suspension cultures derived from a range of explant sources and seedling ages were exposed to variations in the culture medium and changes to the culture environment. None of the treatments tested were successful in promoting plantlet regeneration. Suspension cells were transformed with Agrobacterium tumefaciens strain EHA101 carrying the binary vector pNOV3635 with a gene encoding phosphomannose isomerase (PMI). Transformed callus was selected on medium containing 1–2% mannose. A chlorophenol red assay was used to confirm that the PMI gene was expressed. Polymerase chain reaction and Southern hybridization detected the presence of the PMI gene. Copy number in different lines ranged from one to four.
Similar content being viewed by others
References
Braemer, R.; Paris, M. Biotransformation of cannabinoids by a cell suspension culture of Cannabis saliva L. Plant Cell Rep. 6:150–152; 1987.
Clarke, R. C. Botany of the genus Cannabis. In: Ranalli, P., ed. Advances in hemp research, New York: Hawarth Press: 1999:1–18.
Fisse, J.; Braut, F.; Cosson, L.; Paris, M. Étude in vitro des capacités organogénétiques de tissus de Cannabis sativa L.: effet de différentes substances de croissance. Pl. Méd. Phytoth. 15:217–223; 1981.
Forapani, S.; Carboni, A.; Paoletti, C.; Moliterni, V. M. C.; Ranalli, P.; Mandolino, G. Comparison of hemp varieties using random amplified polymorphic DNA markers. Crop Sci. 41:1682–1689; 2001.
Gamborg, O. L.; Miller, R. A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50:151–158; 1968.
Hartsel, S. C.; Loh, W.H.-T.; Robertson, L. W. Biotransformation of cannabidiol to cannabielsoin by suspension cultures of Cannabis sativa and Saccharum officinarum. Planta Med. 48:17–19; 1983.
Heitrich, A.; Binder, M. Identification of (3R, 4R)-Δ1(6)-tetrahydrocannabinol as an isolation artefact of cannabinoid acids formed by callus cultures of Cannabis sativa L. Experientia 38:898–899; 1982.
Hemphill, J. K.; Turner, J. C.; Mahlberg, P. G. Studies on growth and cannabinoid composition of callus derived from different strains of Cannabis sativa. Lloydia 41:453–462; 1978.
Hood, E. E.; Helmer, G. L.; Fraley, R. T.; Chilton, M.-D. The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J. Bacteriol. 168:1291–1301; 1986.
Itokawa, H.; Takeya, K.; Mihashi, S. Biotransformation of cannabinoid precursors and related alcohols by suspension cultures of callus induced from Cannabis sativa L. Chem. Pharm. Bull. 25:1941–1946; 1977.
Jekkel, Z. S.; Hesky, L. E.; Ali, A. H. Effect of different cryoprotectants and transfer temperatures on the survival rate of hemp (Cannabis sativa L.) cell suspension in deep freezing. Acta Biol. Hung. 40:127–136; 1989.
Joersbo, M. Advances in the selection of transgenic plants using non-antibiotic marker genes. Physiol. Plant. 111:69–272; 2001.
Joersbo, M.; Donaldson, I.; Kreiberg, J.; Petersen, S. G.; Brunstedt, J.; Okkels, F. T. Analysis of mannose selection used for transformation of sugar beet. Mol. Breed. 4:111–117; 1998.
Johnson, P. Industrial hemp: a critical review of claimed potentials for Cannabis sativa. Tappi J. 82:113–123; 1999.
Koetsier, P. A.; Schorr, J.; Doerfler, W. A rapid optimized protocol for downward alkaline Southern blotting of DNA. BioTechniques 15:260–262; 1993.
Kramer, C.; DiMaio, J.; Carswell, G. K.; Shillito, R. D. Selection of transformed protoplast-derived Zea mays colonies with phosphinothricin and a novel assay using the pH indicator chlorophenol red. Planta 190:454–458; 1993.
Loh, W.H.-T.; Hartsel, S. C.; Robertson, L. W. Tissue culture of Cannabis sativa L. and in vitro biotransformation of phenolics. Z. Pflanzenphysiol. 111:S.395–400; 1983.
Lucca, P.; Ye, X.; Potrykus, I. Effective selection and regeneration of transgenic rice plants with mannose as selective agent. Mol. Breed. 7:43–49; 2001.
MacKinnon, L.; McDougall, G.; Aziz, N.; Millam, S. Progress towards transformation of fibre hemp. Scottish Crop Research Institute Annual Report 2000/2001. Invergowrie, Dundee: Scottish Crop Research Institute; 2000:84–86.
Mandolino, G.; Ranalli, P. Advances in biotechnological approaches for hemp breeding and industry. In: Ranalli, P., ed. Advances in hemp research, New York: Haworth Press; 1999:185–208.
Miles, J. S.; Guest, J. R. Nucleotide sequence and transcriptional start point of the phosphomannose isomerase gene (man A) of Escherichia coli. Gene 32:41–48; 1984.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497; 1962.
Negrotto, D.; Jolley, M.; Beer, S.; Wenck, A. R.; Hansen, G. The use of phosphomannose-isomerase as a selectable marker to recover transgenic maize plants (Zea mays L.) via Agrobacterium transformation. Plant Cell Rep. 19:798–803; 2000.
Reed, J.; Privalle, L.; Powell, M. L.; Meghji, M.; Dawson, J.; Dunder, E.; Suttie, J.; Wenck, A.; Launis, K.; Kramer, C.; Chang, Y.-F.; Hansen, G.; Wright, M. Phosphomannose isomerase: an efficient selectable marker for plant transformation. In Vitro Cell. Dev. Biol. Plant 37:127–132; 2001.
Richez-Dumanois, C.; Braut-Boucher, F.; Cosson L.; Paris, M. Multiplication végétative in vitro du chanvre (Cannabis sativa L.). Application à la conservation des clones sélectionnés. Agronomie 6:487–495; 1986.
Sambrook, J.; Fritsch, E. E.; Maniatis, T. Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989.
Schluter, C.; Punja, Z. K. Genetic diversity among natural and cultivated field populations and seed lots of American ginseng (Panax quinquefolius L.) in Canada. Int. J. Plant Sci. 163:427–439; 2002.
Turner, C. E.; Elsohly, M. A.; Boeren, E. G. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J. Nat. Prod. 43:169–237; 1980.
Veliky, I. A.; Genest, K. Growth and metabolites of Cannabis sativa cell suspension cultures. Lloydia 35:450–456; 1972.
Wright, M.; Dawson, J.; Dunder, E.; Suttie, J.; Reed, J.; Kramer, C.; Chang, Y.; Novitzky, R.; Wang, H.; Artim-Moore, L. Efficient biolistic transformation of maize (Zea mays L.) and wheat (Triticum aestivum L.) using the phosphomannose isomerase gene, pmi, as the selectable marker. Plant Cell Rep. 20:429–436; 2001.
Zhang, P.; Potrykus, I.; Puonti-Kaerlas, J. Efficient production of transgenic cassava using negative and positive selection. Trans. Res. 9:405–415; 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feeney, M., Punja, Z.K. Tissue culture and Agrobacterium-mediated transformation of hemp (Cannabis sativa L.). In Vitro Cell Dev Biol -Plant 39, 578–585 (2003). https://doi.org/10.1079/IVP2003454
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2003454