Abstract
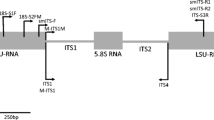
Armillaria species cause economic losses in both pine forests and kiwifruit orchards in New Zealand. In this study Armillaria species-specific PCR primers were developed to facilitate fast and accurate identification of the species found in New Zealand. The first step was to determine which species were present in New Zealand and therefore required distinguishing. Nationally collected Armillaria cultures and specimens had their ribosomal ITS genes sequenced to identify them to species. Sequence data results were consistent with just the four previously recognised species — Armillaria novaezelandiae, A. limonea, A. hinnulea and one as yet unnamed species — being present in New Zealand. Species-specific PCR primers for these four species were subsequently designed targeting the ITS gene region. Specificity of each of the four primer sets for their respective species was confirmed when they were further tested on DNA of the four Armillaria species and other fungi likely to be found in kiwifruit orchards or associated with forest trees.
Similar content being viewed by others
References
Anderson JB, Petsche DM, Smith ML (1987) Restriction fragment polymorphisms in biological species of Armillaria mellea. Mycologia 79, 69–76. doi:10.2307/3807745
Anderson JB, Bailey SS, Pukkila PJ (1989) Variation in ribosomal DNA among biological species of Armillaria, a genus of root-infecting fungi. Evolution 43, 1652–1662. doi:10.2307/2409381
Coetzee MPA, Wingfield BD, Bloomer P, Ridley GS, Kile GA, Wingfield MJ (2001) Phylogenetic relations of Australian and New Zealand Armillaria species. Mycologia 93, 887–896. doi:10.2307/3761754
Coetzee MPA, Wingfield BD, Bloomer P, Ridley GS, Wingfield MJ (2003a) Molecular identification and phylogeny of Armillaria isolates from South America and Indo-Malaysia. Mycologia 95, 285–293. doi:10.2307/3762039
Coetzee MPA, Wingfield BD, Roux J, Crous PW, Denman S, Wingfield MJ (2003b) Discovery of two northern hemisphere Armillaria species on Proteaceae in South Africa. Plant Pathology 52, 604–612. doi:10.1046/j.1365-3059.2003.00879.x
Coetzee MPA, Wingfield BD, Kirisits T, Chhetri DB, Bloomer P, Wingfield MJ (2005) Identification of Armillaria isolates from Bhutan based on DNA sequence comparisons. Plant Pathology 54, 36–45. doi:10.1111/j.1365-3059.2005.01110.x
Dodd SL, Crowhurst RN, Rodrigo AG, Samuels GJ, Hill RA, Stewart A (2000) Examination of Trichoderma phylogenies derived from ribosomal DNA sequence data. Mycological Research 104, 23–34. doi:10.1017/S0953756299001100
Duchesne LC, Anderson JB (1990) Location and direction of transcription of the 5S rRNA gene in Armillaria. Mycological Research 94, 266–269. doi: 10.1016/S0953-7562(09)80626-6
Escofet PE, Aguin O, Mansilla JP (2006) Detection and identification with moleculartechniques of species of the genus Armillaria from soil samples. Boletin de Sanidad Vegetal, Plagas 32, 231–240.
Hanna JW, Klopfenstein NB, Kim MS, McDonald GI, Moore JA (2007) Phylogeographic patterns of Armillaria ostoyae in the western United States. Forest Pathology 37, 192–216. doi:10.1111/j.1439-0329.2007. 00497.x
Hood IA (1989) Armillaria root disease in New Zealand forests. New Zealand Journal of Forestry Science 19, 180–197.
Horner IJ (1991) Epidemiology of Armillaria root-rot of Kiwifruit. Acta Horticulturae 297, 573–578.
Keca N, Bodles WJA, Woodward S, Karadžic D, Bojovič S (2006) Molecular-based identification and phylogeny of Armillaria species from Serbia and Montenegro. Forest Pathology 36, 41–57. doi:10.111 1/j.1439-0329. 2006.00434.x
Kile GA, Watling R (1988) Identification and occurrence of Australian Armillaria species, including A. Pallidula sp. nov. and comparative studies between them and non-Australian tropical and Indian Armillaria. Transactions of the British Mycological Society 91, 305–315. doi:10.1016/S0007-1536(88)80219-5
Kim MS, Klopfenstein NB, McDonald GI, Arumuganathan K, Vidaver AK (2000) Characterisation of North American Armillaria species by nuclear DNA content and RFLP analysis. Mycologia 92, 874–883. doi:10.2307/3761583
Kim MS, Klopfenstein NB, Hanna JW, McDonald GI (2006) Characterization of North American Armillaria species: genetic relationships determined by ribosomal DNA sequences and AFLP markers. Forest Pathology 36, 145–164. doi:10.1111/j.1439-0329.2006.00441.x
Langrell SRH, Lung-Escarmant B, Decroocq S (2001) Isolation and characterization of polymorphic simple sequence repeat loci in Armillaria ostoyae. Molecular Ecology Notes 1, 305–307. doi:10.1046/j.1471-8278.2001.00119.x
Maphosa L, Wingfield BD, Coetzee MPA, Mwenje E, Wingfield MJ (2006) Phylogenetic relationships among Armillaria species inferred from partial elongation factor 1-alpha DNA sequence data. Australasian Plant Pathology 35, 513–520. doi:10.1071/AP06056
McPherson MJ, Moller SG (2006) ‘PCR: the basics.” 2nd edn. (Taylor and Francis Group: New York)
Power MWP, Ramsfield TD, Hood IA (2008) Detection of Armillaria basidiospore dispersal. New Zealand Plant Protection 61, 35–40.
Prodorutti D, Vanblaere T, Gobbin D, Pellegrini A, Gessler C, Pertot I (2009) Genetic diversity of Armillaria spp. infecting Highbush Blueberry in Northern Italy (Trentino Region). Phytopathology 99, 651–658. doi:10.1094/PHYTO-99-6-0651
Ramsfield TD, Power MWP, Ridley GS (2008) A comparison of populations of Armillaria hinnulea in New Zealand and Australia. New Zealand Plant Protection 61, 41–47.
Schulze S, Bahnweg G, Möller EM, Sandermann H (1997) Identification of the genus Armillaria by specific amplification of an rDNA-ITS fragment and evaluation of genetic variation within A. ostoyae by rDNA-RFLP and RAPD analysis. European Journal of Forest Pathology 27, 225–239. doi:10.1111/j.1439-0329.1997.tb00865.x
Sicoli G, Fatehi J, Stenlid J (2003) Development of species-specific PCR primers on rDNA for the identification of European Armillaria species. Forest Pathology 33, 287–297. doi:10.1046/j.1439-0329.2003.00330.x
Smith ML, Anderson JB (1989) Restriction fragment length polymorphisms in mitochondrial DNAs of Armillaria: identification of North American biological species. Mycological Research 93, 247–256. doi:10.1016/S0953-7562(89)80151-0
Smith-White JL, Summerell BA, Gunn LV, Rinzin C, Porter C, Burgess LW (2002) Molecular detection and differentiation of Australian Armillaria species. Australasian Plant Pathology 31, 75–79. doi:10.1071/AP01061
Watling R, Kile GA, Burdsall HH Jr (1991) Nomenclature, taxonomy and identification. In ‘Armillaria root disease’. USDA-Forest Service Agricultural Handbook 691. (Eds CG Shaw, GA Kile) pp. 1–9. (Forest Service, United States Department of Agriculture, Fort Collins, CO, US)
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In ‘PCR protocols: a guide to methods and applications’. (Eds MA Innis, DH Gelfand, JJ Sninsky, TJ White) pp. 315–322. (Academic Press: San Diego, CA)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dodd, S.L., Ramsfield, T.D. & Marshall, J.W. PCR primers to distinguish Armillaria species found in New Zealand. Australasian Plant Pathology 39, 536–543 (2010). https://doi.org/10.1071/AP10026
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1071/AP10026