Abstract
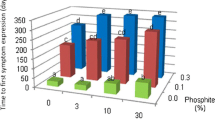
The influence of site on effectiveness of low-volume spray of phosphite for the control of Phytophthora cinnamomi was compared between sites differing in soil nutrient status. The sites ranged from infertile deep grey sands to a red loam in the Esperance Plains bioregion, and infertile gravelly sand of the Havel site-vegetation type P to a red loam of Havel site-vegetation typeQin the northern Jarrah Forest bioregion. Following low-volume spray of 0, 24 or 48 kg phosphite/ha, phosphite effectiveness was determined from assessment of rate of colonisation and inhibition of stem colonisation by P. cinnamomi, and stem phosphite concentration in challenge inoculation stems of Lambertia inermis var. inermis in Esperance Plains bioregion sites and Banksia grandis in Jarrah Forest bioregion sites. Phosphite effectiveness was mainly influenced by plant species rather than site. Phosphite spray significantly controlled P. cinnamomi colonisation in B. grandis, but not in L. inermis var. inermis. Site had no consistent influence on the effect of phosphite on P. cinnamomi despite large differences in soil nutrient status between sites. Differences between sites and site ranking changed with inoculation time and rate of phosphite spray, and duplicate sites differed significantly from each other. Site, rate of phosphite spray, time after spray and plant species significantly affected stem phosphite concentrations. In both Esperance Plains bioregion and Jarrah Forest bioregion sites, stem phosphite concentrations were greatest in fertile loam sites and least in infertile sand or gravelly sand sites. Stem phosphite concentrations increased with rate of phosphite spray with concentrations in stems receiving 48 kg phosphite/ha being 1.2 to 3-times-greater than that in stem sprayed with 24 kg phosphite/ha. Six months after low-volume spray, phosphite concentrations in L. inermis var. inermis stems had declined for all sites and spray rates to only 4–17% of original concentrations. Decline of phosphite concentrations in B. grandis stems was slower than that for L. inermis var. inermis stems. The results indicate that effectiveness of phosphite against P. cinnamomi infection in different communities will depend more on plant species composition than soil nutrient status. Differences in phosphite effectiveness between plant species may be related to differences in phosphite concentration thresholds yet to be quantified, above which tissue concentrations must be achieved before inhibition of P. cinnamomi colonisation occurs. In rapid colonisation/phosphite low-responsive species such as L. inermis var. inermis, the threshold may be greater than that required for a response in a rapid colonisation/phosphite high-response species such as B. grandis. These results demonstrate the need to determine thresholds for individual plant species and in different environments.
Similar content being viewed by others
References
Afek U, Sztejnberg A (1989) Effects of Fosetyl-Al and phosphorus acid on scoparone, a phytoalexin associated with resistance of citrus to Phytophthora citrophthora. Phytopathology 79, 736–739. doi: 10.1094/Phyto-79-736
Barchietto T, Saindrenan P, Bompeix G (1988) Characterization of phosphite uptake in two Phytophthora spp. and its inhibition by phosphate. Archives of Microbiology 151, 54–58. doi: 10.1007/BF00444669
Barrett SR (2001) Phytotoxic effects of phosphite in native plant communities in southern Western Australia. Ph.D. Thesis, Murdoch University.
Barrett S (2003) Monitoring of aerial phosphite applications for the control of Phytophthora cinnamomi in the Albany district. In ‘Phytophthora in forests and natural ecosystems. 2nd International IUFRO Working Party 7.02.09 Meeting, Albany, W. Australia’. (Eds JA McComb, GE StJ Hardy, IC Tommerup) pp. 132–137. (Murdoch University Print, Murdoch)
Barrett SR, Shearer BL, Hardy GEStJ (2002) Root and shoot development in Corymbia calophylla and Banksia brownii after the application of the fungicide phosphite. Australian Journal of Botany 50, 155–161. doi: 10.1071/BT01018
Boesewinkel HJ (1976) Storage of fungal cultures in water. Transactions of the British Mycological Society 66, 183–185.
Carswell C, Grant BR, Theodorou ME, Harris J, Niere JO, Plaxton WC (1996) The fungicide phosphonate disrupts the phosphate-starvation response in Brassica nigra seedlings. Plant Physiology 110, 105–110.
Cochrane JA, Crawford AD, Monks LT (2007) The significance of ex situ seed conservation to reintroduction of threatened plants. Australian Journal of Botany 55, 356–361. doi: 10.1071/BT06173
Day PR (1965) Particle fraction and particle-size analysis. In ‘Methods of soil analysis. Part 1’. Agronomy Monograph 9 (Ed. CA Black) pp. 545–567. (ASA and SSSA: Madison Wisconsin)
El-Hamalawi ZA, Menge JA, Adams CJ (1995) Methods of Fosetyl-Al application and phosphonate levels in avocado tissue needed to control stem canker caused by Phytophthora citricola. Plant Disease 79, 770–778.
Foulds W (1993) Nutrient concentrations of foliage and soil in south-western Australia. The New Phytologist 125, 529–546. doi: 10.1111/j.1469-8137.1993.tb03901.x
Guest D, Grant B (1991) The complex action of phosphonates as antifungal agents. Biological Reviews of the Cambridge Philosophical Society 66, 159–187. doi: 10.1111/j.1469-185X.1991.tb01139.x
Havel JJ (1975) Site-vegetation mapping in the northern jarrah forest (Darling Range). Definition of site-vegetation types. Bulletin[nu 86. (Forests Department: Perth)
Jackson TJ, Burgess T, Colquhoun I, Hardy GEStJ (2000) Action of the fungicide phosphite on Eucalyptus marginata inoculated with Phytophthora cinnamomi. Plant Pathology 49, 147–154. doi: 10.1046/ j.1365-3059.2000.00422.x
Kirby KN (1993) ‘Advanced data analysis with SYSTAT.’ (Van Nostrad Reinhold: New York).
Komorek B, Shearer BL, Smith B, Fairman RG (1997) The control of Phytophthora in native plant communities. In ‘Control of Phytophthora and Diplodina Canker in Western Australia. Final Report to the Threatened Species and Communities Biodiversity Group, Environment Australia’. (Ed. D Murray) pp. 1–59. (Department of Conservation and Land Management: Perth)
Lamont BB (1995) Mineral nutrient relations in Mediterranean regions of California, Chile, and Australia. In ‘Ecology and biogeography of Mediterranean ecosystems in Chile, California, and Australia.’ (Eds MT Kalin Arroyo, PH Zedler, MD Fox) pp. 211–235. (Springer-Verlag: New York)
McKenzie HA, Wallace HS (1954) The Kjeldahl determination of nitrogen: a critical study of digestion conditions - temperature, catalyst and oxidising agent. Australian Journal of Chemistry 7, 55–70.
Mead R (1988) ‘The design of experiments: statistical principles for practical application’. (Cambridge University Press: Cambridge).
Monks L, Coates D (2002) The translocation of two critically endangered Acacia species. Conservation Science Western Australia 4, 54–61.
Moran MD (2003) Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100, 403–405. doi: 10.1034/j.1600-0706.2003.12010.x
Murphy J, Riley T (1962) A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27, 31–36. doi: 10.1016/S0003-2670(00)88444-5
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Ouimette DG, Coffey MD (1990) Symplastic entry and phloem translocation of phosphonate. Pesticide Biochemistry and Physiology 38, 18–25. doi: 10.1016/0048-3575(90)90143-P
Pilbeam RA, Colquhoun IJ, Shearer B, Hardy GEStJ (2000) Phosphite concentration: its effect on phytotoxicity symptoms and colonisation by Phytophthora cinnamomi in three understorey species in Eucalyptus marginata forest. Australasian Plant Pathology 29, 86–95. doi: 10.1071/AP00016
Piper CS (1942) ‘Soil and plant analysis’ (University of Adelaide: Adelaide)
Schoknecht N (2002) Soil groups of Western Australia. A simple guide to the main soils of Western Australia. Resource Management Technical Report 246. (Department of Agriculture: South Perth)
Shea SR, Gillen KJ, Leppard WI (1980) Seasonal variation in population levels of Phytophthora cinnamomi Rands in soil in diseased, freely drained Eucalyptus marginata Sm sites in northern jarrah forest of south-western Australia. Protection Ecology 2, 135–156.
Shearer BL, Crane CE (2003) The influence of soil from a topographic gradient in the Fitzgerald River National Park on mortality of Banksia baxteri following infection by Phytophthora cinnamomi. In ‘Phytophthora in Forests and Natural Ecosystems. 2nd International IUFRO Working Party 7.02.09 Meeting, Albany, W. Australia’. (Eds JA McComb, GE StJ Hardy, IC Tommerup) pp. 268. (Murdoch University Print, Murdoch)
Shearer BL, Fairman RG (2007a) A stem injection of phosphite protects Banksia species and Eucalyptus marginata from Phytophthora cinnamomi for at least four years. Australasian Plant Pathology 36, 78–86. doi: 10.1071/AP06085
Shearer BL, Fairman RG (2007b) Application of phosphite in a high-volume foliar spray delays and reduces the rate of mortality of four Banksia species infected with Phytophthora cinnamomi. Australasian Plant Pathology 36, 358–368. doi: 10.1071/AP07033
Shearer BL, Smith IW (2000). Diseases of eucalypts caused by soilborne species of Phytophthora and Pythium. In ‘Diseases and Pathogens of Eucalypts’. (Eds PJ Keane, GA Kile, FD Podger, BN Brown) pp. 259–291. (CSIRO Publishing: Canberra)
Shearer BL, Tippett JT (1989) Jarrah dieback: The Dynamics and Management of Phytophthora cinnamomi in the Jarrah (Eucalyptus marginata) Forest of South-western Australia. Department of Conservation and Land Management Research Bulletin 3.
Shearer BL, Crane CE, Cochrane A (2004a) Quantification of the susceptibility of the native flora of the South-West Botanical Province, Western Australia, to Phytophthora cinnamomi. Australian Journal of Botany 52, 435–443. doi: 10.1071/BT03131
Shearer BL, Crane CE, Fairman RG (2004b) Phosphite reduces disease extension of a Phytophthora cinnamomi front in Banksia woodland, even after fire. Australasian Plant Pathology 33, 249–254. doi: 10.1071/AP04002
Shearer BL, Fairman RG, Grant MJ (2006) Effective concentration of phosphite in controlling Phytophthora cinnamomi following stem injection of Banksia species and Eucalyptus marginata. Forest Pathology 36, 119–135. doi: 10.1111/j.1439-0329.2006.00440.x
Shearer BL, Michaelsen BJ, Somerford PJ (1988) Effects of isolate and time of inoculation on invasion of secondary phloem of Eucalyptus spp. and Banksia grandis by Phytophthora spp. Plant Disease 72, 121–126. doi: 10.1094/PD-72-0121
Shearer BL, Michaelsen BJ, Warren HJ (1987a) Comparative behaviour of Phytophthora species in the secondary phloem of stems and excised roots of Banksia grandis and Eucalyptus marginata. Australian Journal of Botany 35, 103–110. doi: 10.1071/BT9870103
Shearer BL, Shea SR, Deegan PM (1987b) Temperature-growth relationships of Phytophthora cinnamomi in the secondary phloem of roots of Banksia grandis and Eucalyptus marginata. Phytopathology 77, 661–665. doi: 10.1094/Phyto-77-661
Shearer BL, Crane CE, Barrett S, Cochrane A (2007a) Phytophthora cinnamomi invasion, a major threatening process to conservation of flora diversity in the South-West Botanical Province of Western Australia. Australian Journal of Botany 55, 225–238. doi: 10.1071/BT06019
Shearer BL, Crane CE, Barrett S, Cochrane A (2007b) Assessment of threatened flora susceptibility to Phytophthora cinnamomi by analysis of disease progress curves in shadehouse and natural environments. Australasian Plant Pathology 36, 609–620. doi: 10.1071/AP07074
Smillie R, Grant BR, Guest D (1989) The mode of action of phosphite: evidence for both direct and indirect modes of action on three Phytophthora spp. in plants. Phytopathology 79, 921–926. doi: 10.1094/Phyto-79-921
Tippett JT, Crombie DS, Hill TC (1987) Effect of phloem water relations on the growth of Phytophthora cinnamomi in Eucalyptus marginata. Phytopathology 77, 246–250. doi: 10.1094/Phyto-77-246
Tippett JT, Hill TC, Shearer BL (1985) Resistance of Eucalyptus spp. to invasion by Phytophthora cinnamomi. Australian Journal of Botany 33, 409–418. doi: 10.1071/BT9850409
Tippett JT, McGrath JF, Hill TC (1989) Site and seasonal effects on susceptibility of Eucalyptus marginata to Phytophthora cinnamomi. Australian Journal of Botany 37, 481–490. doi: 10.1071/BT9890481
Tsao PH, Guy SO (1977) Inhibition of Mortierella and Pythium in Phytophthora-isolation medium containing hymexazol. Phytopathology 67, 796–801.
Tynan KM, Wilkinson CJ, Holmes JM, Dell B, Colquhoun IJ, McComb JA, Hardy GEStJ (2001) The long-term ability of phosphite to control Phytophthora cinnamomi in two native plant communities of Western Australia. Australian Journal of Botany 49, 761–770. doi: 10.1071/BT00062
Whiley AW, Hargreaves PA, Pegg KG, Doogan VJ, Ruddle LJ, Saranah JB, Langdon PW (1995) Changing sink strengths influence translocation of phosphonate in avocado (Persea americana Mill.) trees. Australian Journal of Agricultural Research 46, 1079–1090. doi: 10.1071/AR9951079
Wilkinson CJ, Holmes JM, Tynan KM, Colquhoun IJ, McComb JA, Hardy GEStJ, Dell B (2001) Ability of phosphite applied in a glasshouse trial to control Phytophthora cinnamomi in five plant species native to Western Australia. Australasian Plant Pathology 30, 343–351. doi: 10.1071/AP01055
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shearer, B.L., Crane, C. Influence of site and rate of low-volume aerial phosphite spray on lesion development of Phytophthora cinnamomi and phosphite persistence in Lambertia inermis var. inermis and Banksia grandis . Australasian Plant Pathology 38, 288–304 (2009). https://doi.org/10.1071/AP09005
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1071/AP09005