Abstract
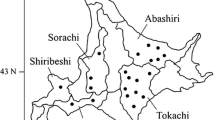
A unique sampling strategy was used to search for genetic similarity among isolates of Rhynchosporium secalis, the causal agent of leaf scald of barley. A field of barley cv. Skiff with discrete ‘hotspots’ of leaf scald was identified and infected leaves were collected for this study. The isolates were genotyped using amplified fragment length polymorphism (AFLP) analysis. In contrast to previous reports of high diversity of R. secalis isolates in areas of only 1 m2, we found close genetic similarity between isolates from the same hotspot as determined by AFLP marker alleles, with a higher level of variation between isolates from different hotspots. A UPGMA phenogram showed that most isolates clustered with members of the same hotspot. This pattern is consistent with the inoculum source for the hotspots being airborne ascospores or wind-dispersed conidia from a local founder population, borne in splash-created aerosols. AFLP variation within hotspots suggests a high rate of mutation within a few cycles of fungal infection. A collection of isolates from cv. Skiff plots in a straw-inoculated disease nursery 60 km from the hotspot field was fingerprinted with AFLPs for comparison and was found to have much greater genotypic diversity.
Similar content being viewed by others
References
Ali SM, Mayfield AH, Clare BG (1976) Pathogenicity of 203 isolates of Rhynchosporium secalis on 21 barley cultivars. Plant Pathology 9, 153–443.
Armstrong JS, Gibbs AJ, Peakall R, Weiler G (1994) ‘The RAPDistance package’ http://life.anu.edu.au/molecular/software/rapd.html
Ayesu-Offei EN, Carter MV (1971) Epidemiology of leaf scald of barley. Australian Journal of Agricultural Research 22, 383–390.
Baayen RP, O'Donnell K, Bonants PJM, Cigelnik E, Kroon LPNM, Roebroeck EJA, Waalwijk C (2000) Gene genealogies and AFLP analyses in the Fusarium oxysporum complex identify monophyletic and nonmonophyletic formae speciales causing wilt and root disease. Phytopathology 90, 891–900.
Burdon JJ, Abbott DC, Brown AHD, Brown JS (1994) Genetic structure of the scald pathogen (Rhynchosporium secalis) in South East Australia: implications for control strategies. Australian Journal of Agricultural Research 45, 1145–1154.
Ceoloni C (1980) Race differentiation and search for sources of resistance to Rhynchosporium secalis in barley in Italy. Euphytica 29, 547–553.
Evans SG (1969) Observations on the development of leaf blotch and net blotch of barley from barley debris. Plant Pathology 18, 116–118.
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131, 479–491.
Farman ML, Taura S, Leong SA (1996) The Magnaporthea grisea DNA fingerprinting probe MGR586 contains the 3′ end of an inverted repeat transposon. Molecular and General Genetics 251, 675–681.
Felsenstein J (1993) PHYLIP—Phylogeny Inference Package Version 3.5c.
Fowler AM, Owen H (1971) Studies on leaf blotch of barley (Rhynchosporium secalis). Transactions of the British Mycological Society 56, 137–152.
Goodwin SB, Allard RW, Hardy SA, Webster RK (1992) Heirarchical structure of pathogen variation among Rhynchosporium secalis populations in Idaho and Oregon. Canadian Journal of Botany 70, 810–817.
Goodwin SB, Cavaletto JR, Waalwijk C, Kema GHJ (2001) DNA fingerprint probe from Mycosphaerella graminicola identifies an active transposable element. Phytopathology 91, 1181–1188.
Goodwin SB, Saghai Maroof MA, Allard RW, Webster RK (1993) Isozyme variation within and among populations of Rhynchosporium secalis in Europe, Australia and the United States. Mycological Research 97, 49–58.
Goodwin SB, Webster RK, Allard RW (1994) Evidence for mutation and migration as sources of genetic variation in populations of Rhynchosporium secalis. Phytopathology 84, 1047–1053.
Jaccard P (1901) Etude comparative de la distribution florale dans une portion des Alpes et des Jura. Bulletin de la Société Vaudoise des Sciences Naturelles 37, 547–579.
Jaccard P (1908) Nouvelles recherches sur la distribution florale. Bulletin de la Société Vaudoise des Sciences Naturelles 44, 223–270.
Jackson LF, Kahler AL, Webster RK, Allard RW (1978) Conservation of scald resistance in barley composite cross populations. Phytopathology 68, 645–650.
Khan TN (1986) Effect of fungicide treatment of scald (Rhynchosporium secalis (Oud) J Davis) infection on some quality characteristics of barley. Australian Journal of Experimental Agriculture 28, 783–785.
Majer D, Mithen R, Lewis BG, Vos P, Oliver RP (1996) The use of AFLP fingerprinting for the detection of genetic variation in fungi. Mycological Research 100, 1107–1111
McDermott JM, McDonald BA, Allard RW, Webster RK (1989) Genetic variability for pathogenicity, isozyme, ribosomal DNA and colony colour variants in populations of Rhynchosporium secalis. Genetics 122, 561–565.
McDonald BA, Linde C (2002) Pathogen population genetics, evolutionary potential and durable resistance. Annual Review of Phytopathology 40, 349–379.
McDonald BA, Zhan J, Burdon JJ (1999) Genetic structure of Rhynchosporium secalis in Australia. Phytopathology 89, 639–645.
Newman PL, Owen H (1985) Evidence of asexual recombination in Rhynchosporium secalis. Plant Pathology 34, 338–340.
Newton AC (1989) Somatic recombination in Rhynchosporium secalis. Plant Pathology 38, 71–74.
Newton AC, Searle J, Guy DC, Hackett CA, Cooke DEL (2001) Variability in pathotype, aggressiveness, RAPD profile, and rDNA ITS1 sequences of UK isolates of Rhynchosporium secalis. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz 108, 446–458.
O'Hanlon PC, Peakall R (2000) A simple method for the detection of size homoplasy among amplified fragment length polymorphism fragments. Molecular Ecology 9, 815–816.
Parsons YM, Shaw KL (2001) Species boundaries and genetic diversity among Hawaiian crickets of the genus Laupala identified using amplified fragment length polymorphism. Molecular Ecology 10, 1765–1772.
Pringle RB, Sheffer RP (1963) Purification of the selective toxin of Periconia circinata. Phytopathology 53, 758–787
Raeder U, Broda P (1985) Rapid preparation of DNA from filamentous fungi. Letters in Applied Microbiology 1, 17–20.
Salamati S, Zhan J, Burdon JJ, McDonald BA (2000) The genetic structure of field populations of Rhynchosporium secalis from three continents suggests moderate gene flow and regular recombination. Phytopathology 90, 901–908.
Shipton WA, Boyd WJR, Ali SM (1974) Scald of barley. Review of Plant Pathology 53, 839–861.
Stedman OJ (1980) Observations on the production and dispersal of spores, and infection by Rhynchosporium secalis. Annals of Applied Biology 95, 163–175.
Tekauz A (1991) Pathogenic variation in Rhynchosporium secalis on barley in Canada. Canadian Journal of Plant Pathology 13, 298–304.
Vos P, Hogers R, Bleeker M, Reijans M, van der Lee T, Homes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Research 23, 4407–4414.
Williams KJ, Smyl C, Lichon A, Wong KY, Wallwork H (2001) Development and use of a PCR-based assay that differentiates the pathogens causing spot form and net form of net blotch of barley. Australasian Plant Pathology 30, 37–44.
Yap IV, Nelson RJ (1996) ‘Winboot: a program for performing bootstrap analysis of binary data to determine the confidence limits of UPGMA-based dendrograms.’ (International Rice Research Institute: Manila, Philippines)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, K., Donnellan, S., Smyl, C. et al. Molecular variation in Rhynchosporium secalis isolates obtained from hotspots. Australasian Plant Pathology 32, 257–262 (2003). https://doi.org/10.1071/AP03008
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1071/AP03008