Abstract
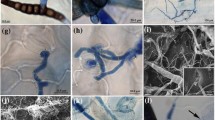
A variety of reactions to inoculation with Phytophthora cinnamomi ranging from high susceptibility to moderate resistance were found in 20 ecotypes of Arabidopsis thaliana. P. cinnamomi zoospores successfully colonised both root and leaf tissue of Arabidopsis and sporulation in the form of chlamydospores and sporangia occurred in leaves and roots of each ecotype but the number varied considerably between ecotypes. In the more susceptible ecotypes, colonisation was characterised by rapid intercellular growth and sporulation of the pathogen from 48 h post inoculation. In less susceptible ecotypes, P. cinnamomi was limited to a defined region within tissues. In response to P. cinnamomi infection, several ecotypes expressed active defence responses in both root and leaf tissue. Callose formation was close ly associated with lesion restriction as was the production of the reactive oxygen species, hydrogen peroxide. The oxidative burst was not limited to the site of pathogen ingress but also occurred in distant, uninfected tissues. We have characterised an Arabidopsis—P. cinnamomi system that will be useful for further studies of active resistance mechanisms.
Similar content being viewed by others
References
Able AJ, Guest DI, Sutherland MW (1998) Use of a new tetrazolium-based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores of Phytophthora parasitica var. nicotianae. Plant Physiology 117, 491–499.
Adam I, Somerville SC (1996) Genetic characterisation of five powdery mildew resistance loei in Arabidopsis thaliana. The Plant Journal 9, 341–356.
Alexander D, Goodman RM, Gut-Rella M, Glascock C, Weymann K, Friedrich L, Maddox D, Ahl-Goy P, Luntz T, Ward E, Ryals J (1993) Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis-related protein la. Proceedings of the National Academy of Sciences USA 90, 7327–7331.
Alonso-Blanco C, Koornneef M (2000) Naturally occurring variation in Arabidopsis: an underexploited resource for plant genetics. Trends in Plant Science 5, 22–29.
Alvarez ME, Pennell RI, Meijer P-L, Ishikawa A, Dixon RA, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92, 773–784.
Apostol I, Heinstein PF, Low PS (1989) Rapid stimulation of an oxidative burst during elicitation of cultured plant cells. Plant Physiology 90, 109–116.
Baker CJ, Orlandi EW (1995) Active oxygen in plant pathogenesis. Annual Review of Phytopathology 33, 299–321.
Bent AF (1996) Plant disease resistance genes: function meets structure. The Plant Cell 8, 1757–1771.
Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, Dong X (1994) A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. The Plant Cell 6, 1845–1857.
Buell RC (1998) Arabidopsis: a weed leading the field of plant-pathogen interactions. Plant Physiology and Biochemistry 36, 177–186.
Byrt PY, Grant BR (1979) Some conditions governing zoospore production in axenic cultures of Phytophthora cinnamomi Rands. Australian Journal of Botany 27, 103–115.
Cahill DM, Bennett IJ, McComb JA (1992) Resistance of micropropagated Eucalyptus marginata to Phytophthora cinnamomi. Plant Disease 76, 630–632.
Cahill DM, Legge N, Grant BR, Weste GM (1989) Cellular and histological changes induced by Phytophthora cinnamomi in a group of plant species ranging from fully susceptible to fully resistant. Phytopathology 79, 417–424
Cahill DM, Weste G, Grant BR (1986) Changes in cytokinin concentrations in xylem extrudate following infection of Eucalyptus marginata with Phytophthora cinnamomi Rands. Plant Physiology 81, 1103–1109.
Dangl JL, Dietrich RA, Richberg MH (1996) Death don’t have no mercy: cell death programs in plant-microbe interactions. The Plant Cell 8, 1793–1807.
Davis KR (1993). Arabidopsis as a model plant system. In ‘Arabidopsis thaliana as a model for plant-pathogen interactions’. (Eds KR Davis, R Hammerschmidt) pp. 1–3. (The American Phytopathological Society Press: St Paul)
Dawson PD, Weste GM (1984) Impact of root infection by Phytophthora cinnamomi on the water relations of two Eucalyptus species that differ in susceptibility. Phytopathology 74, 486–490
Dietrich RA, Delany TP, Uknes SJ, Ward ER, Ryals JA, Dangl JL (1994) Arabidopsis mutants simulating disease resistance response. Cell 77, 565–577.
Doke N (1983) Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissue to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiological Plant Pathology 23, 345–357.
Eschrich W, Currier HB (1964) Identification of callose by its diachrome and fluorochrome reactions. Stain Technology 39, 303–307.
Fuchs H, Sacristián MD (1996) Identification of a gene in Arabidopsis thaliana controlling resistance to clubroot (Plasmodiophora brassicae) and characterisation of the resistance response. Molecular Plant-Microbe Interactions 9, 91–97.
Hoagland DR, Arnon DI (1950) ‘The water culture method for growing plants without soil.’ California Agriculture Experimental Station Circular No. 347.
Irwin JAG, Cahill DM, Drenth A (1995) Phytophthora in Australia. Australian Journal of Agricultural Research 46, 1311–1337.
Kamoun S, Huitema E, Vleeshouwers VGAA (1999) Resistance to oomycetes: a general role for the hypersensitive response? Trends in Plant Science 4, 196–200.
Kellam MK, Coffey MD (1985) Quantitative comparison of the resistance to Phytophthora root rot in three avocado rootstocks. Phytopathology 75, 230–234
Lee S, Stenger DC, Bisaro DM, Davis KR (1994) Identification of loei in Arabidopsis that confer resistance to geminivirus infection. The Plant Journal 6, 525–535.
Manners JG (1993) ‘Principles of plant pathology (2nd edn).’ pp. 143–152. (Cambridge University Press: Cambridge)
Mehdy MC (1994) Active oxygen species in plant defence against pathogens. Plant Physiology 105, 467–472.
Miller PM (1955) V-8 juice agar as a general purpose medium for fungi and bacteria. Phytopathology 45, 461–462.
Osbourn AE (1996) Preformed antimicrobial compounds and plant defence against fungal attack. The Plant Cell 8, 1821–1831.
Phillips D, Grant BR, Weste GM (1984) Histological changes in roots of an avocado cultivar, Duke 7, infected with Phytophthora cinnamomi. Phytopathology 77, 691–698
Price RA, Palmer JD, Al-Shehbaz IA (1994) Systematic relationships of Arabidopsis: a molecular and morphological perspective. In ‘Arabidopsis’. pp. 7–19. (Cold Spring Harbor Laboratory Press: USA)
Roetschi A, Si-Ammour A, Belbahri L, Mauch F, Mauch-Mani B (2001) Characterisation of an Arabidopsis-Phytophthora pathosystem: resistance requires a functional PAD2 gene and is independent ofsalicylic acid, ethylene andjasmonic acid signalling. The Plant Journal 28, 293–305.
Siegrist J, Orober M, Buchenauer H (2000) Beta-aminobutyric acid-mediated enhancement of resistance in tobacco to tobacco mosaic virus depends on the accumulation of salicylic acid. Physiological and Molecular Plant Pathology 56, 95–106
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localisation of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. The Plant Journal 11, 1187–1194.
Tor M, Gordon P, Cuzick A, Eulgem T, Sinapidou E, Mert-Turk F, Can C, Dangl JL, Holub EB (2002) Arabidopsis SGTlb is required for defense signaling conferred by several downy mildew resistance genes. The Plant Cell 14, 993–1003.
Uknes S, Mauch-Mani B, Moyer M, Potter S, Williams S, Dincher S, Chandler D, Slusarenko A, Ward E, Ryals J (1992) Acquired resistance in Arabidopsis. The Plant Cell 4, 645–656.
Venisse JS, Gullner G, Brisset MN (2001) Evidence for the involvement of an oxidative stress in the initiation of infection of pear by Erwinia amylovora. Plant Physiology 125, 2164–2172.
Vogel J, Somerville S (2000) Isolation and characterisation of powdery mildew-resistant Arabidopsis mutants. Proceedings of the National Academy of Sciences USA 97, 1897–1902.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robinson, L.H., Cahill, D.M. Ecotypic variation in the response of Arabidopsis thaliana to Phytophthora cinnamomi . Australasian Plant Pathology 32, 53–64 (2003). https://doi.org/10.1071/AP02064
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1071/AP02064