Abstract
Background
Measurements of the rate of myocardial glucose utilization (rMGU) play a key role in the assessment of alterations in myocardial substrate metabolism in normal and abnormal cardiac states. In this study we determined whether rMGU could be quantified by positron emission tomography (PET) and 1-carbon-11-glucose.
Methods and Results
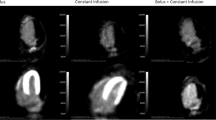
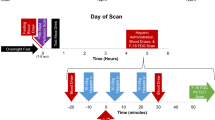
Twenty dogs were studied with a variety of interventions including fasting (n = 5), hyperinsulinemic-euglycemic clamp at rest (n = 6), clamp and phenylephrine (n = 5), and clamp and dobutamine (n = 4). Measurements of myocardial blood flow and rMGU were made by PET with oxygen-15-water and 1-C-11-glucose, respectively. Arterial-coronary sinus sampling was performed to measure rMGU by the Fick method. Values for rMGU ranged from 50 to 2436 nmol/g/min. Myocardial 1-C-11-glucose images of high quality were obtained. There was a close and direct correlation between values for rMGU measured by PET and those measured directly (y = 0.86x + 112, r = 0.98, P < .0001). The coefficient of variation for the regional estimates of rMGU ranged from 11.3% ± 7.4% during clamp at rest to 16.3% ± 8.4% during clamp with phenylephrine.
Conclusions
It now appears possible to quantify myocardial glucose utilization by PET with 1-C-11-glucose. This method should become a valuable tool in the assessment of alterations in myocardial glucose metabolism in both normal and abnormal myocardium.
Similar content being viewed by others
References
Ratib O, Phelps ME, Huang S-C, Henze E, Selin CE, Schelbert HR. The deoxyglucose method for the estimation of local myocardial glucose metabolism with positron computed tomography. J Nucl Med 1982;23:577–86.
Krivokapich J, Huang SC, Phelps ME, Barrio JR, Watanabe CR, Selin CE, et al. Estimation of rabbit myocardial metabolic rate for glucose using fluorodeoxyglucose. Am J Physiol 1982;243:H884–95.
Gambhir SS, Schwaiger M, Huang S-C, Krivokapich J, Schelbert HR, Nienaber CA, et al. Simple noninvasive quantification method for measuring myocardial glucose utilization in humans employing positron emission tomography and fluorine-18 deoxyglucose. J Nucl Med 1989;30:359–66.
Choi Y, Hawkins RA, Huang S-C, Gambhir SS, Brunken RC, Phelps ME, et al. Parametric images of myocardial metabolic rate of glucose generated from dynamic cardiac PET and 2-[18F]fluoro-2-deoxy-D-glucose studies. J Nucl Med 1991;32:733–8.
Hariharan R, Bray M, Ganim R, Doenst T, Goodwin GW, Taegtmeyer H. Fundamental limitations of [18F]2-deoxy-2-fluoro-D-glucose for assessing myocardial glucose uptake. Circulation 1995;91:2435–44.
Marshall RC, Powers-Risius P, Huesman RH, Reutter BW, Taylor SE, Maurer HE, et al. Estimating glucose metabolism using glucose analogs and two tracer kinetic models in isolated rabbit heart. Am J Physiol 1998;44:H668–79.
Botker HE, Goodwin GW, Holden JE, Doenst T, Gjedde A, Taegtmeyer H. Myocardial glucose uptake measured with fluorodeoxyglucose: a proposed method to account for variable lumped constants. J Nucl Med 1999;40:1186–96.
Stone-Elander S, Halldin C, Langstrom B, Blomqvist G, Hamnqvist F, Printz G, et al. Method for routine production of 1-11C-D-glucose from 11C-ammonium cyanide. J Nucl Med 1989;5:927–31.
Dence CS, Powers WJ, Welch MJ. Improved synthesis of 1-11C-d-glucose. Appl Radiat Isot 1993;44:971–80.
Hawkins, Mans AM, Davis DW, Vina JR, Hibbard LS. Cerebra glucose use measured with 14C-glucose labeled in 1, 2, or 6 position. Am J Physiol 1985;248:C170–6.
Blomqvist G, Stone-Elander S, Halldin C, Roland PE, Widen L, Lindqvist M, et al. Positron emission tomographic measurements of cerebral glucose utilization using 1-11C-D-glucose. J Cereb Blood Flow Metab 1990;10:467–83.
Blomqvist G, Stone-Elander S, Halldin C, Roland PE, Swahn CG, Haaparanta M, et al. Cerebral glucose utilization measured with positron emission tomography: 1-11C-D-glucose compared with 2-18F-2-fluoro-deoxy-D-glucose. Acta Radiologica 1991;376:171–2.
Powers WJ, Dagago-Jack S, Markham J, Larson KB, Dence CS. Cerebral transport and metabolism of 1-11C-D-glucose during stepped hypoglycemia. Ann Neurol 1995;38:599–608.
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–31.
Bergmann SR, Herrero P, Matkham J, Weinheimer CJ, Walsh MN. Noninvasive quantitation of myocardial blood flow in human subjects with oxygen-15-labeled water and positron emission tomography. J Am Coll Cardiol 1989;14:639–52.
Herrero P, Markham J, Bergmann SR. Quantitation of myocardial blood flow with H2 15O and positron emission tomography: assessment and error analysis of a mathematical approach. J Comput Assist Tomogr 1989;13:862–73.
Herrero P, Hartmann JJ, Senneff MJ, Bergmann SR. Effects of time discrepancies between input and myocardial time-activity curves on estimates of regional myocardial perfusion with PET. J Nucl Med 1994;35:558–66.
Lund-Andersen H. Transport of glucose from blood to brain. Physiol Rev 1979;59:304–52.
Gear CW. Numerical initial-value problems in ordinary differential equations. Englewood Cliffs (NJ): Prentice-Hall; 1971.
Dennis JE, Schnabel RB. Numerical methods for unconstrained optimization and nonlinear equations. Englewood Cliffs (NJ): Prentice-Hall; 1983.
Lifton JF, Welch MJ. Preparation of glucose labeled with 20-min half-lived carbon-11. Radiat Res 1971;45:35–40.
Mintun MA, Raichle ME, Welch MJ, et al. Brain glucose metabolism measured by PET and U-11C-glucose. J Cereb Blood Flow Metab 1985;5(suppl):623–4.
Gutniak M, Blomqvist G, Widen L, Stone-Elander S, Hamberger B, Grill V. D-[U-11C]glucose uptake and metabolism in brain of insulindependent diabetic subjects. Am J Physiol 1990;258:E805–12.
Sacks W. Cerebral metabolism of doubly labeled glucose in humans in vivo. J Appl Physiol 1965;20:117–130.
Goodwin GW, Ahmad F, Doenst T, Taegtmayer H. Energy provision from glycogen, glucose, and fatty acids on adrenergic stimulation of isolated working rat hearts. Am J Physiol 1998;274:H1239–47.
Collins-Nakai RL, Noseworthy D, Lopaschuk GD. Epinephrine increases AT P production in hearts by preferentially increasing glucose metabolism. Am J Physiol 1994;267:H1862–71.
Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation 1999;99:578–88.
Brooks GA. Intra- and extra-cellular lactate shuttles. Med Sci Sports Exerc 2000;32:790–9.
Bing RJ. The metabolism of the heart. Harvey Lect 1955;50:27–70.
Neely JR, Morgan HE. Relationship between carbohydrate metabolism and energy balance of heart muscle. Annu Rev Physiol 1974;36:413–59.
Zimmer HG. Regulation of and intervention into the oxidative pentose phosphate pathway and adenine nucleotide metabolism in the heart. Mol Cell Biochem 1996;160-161:101–9.
McNulty PH, Jagasia D, Cline GW, Ng CK, Whiting JM, Garg P, et al. Persistent changes in myocardial glucose metabolism in vivo during reperfusion of a limited-duration coronary occlusion. Circulation 2000;10:917–28.
Maclean DA, Ettinger SM, Sinoway LI, Lanoue KF. Determination of muscle-specific glucose flux using radioactive stereoisomers and microdialysis. Am J Physiol 2001;280:E187–92.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by NIH grant HL13581.
Rights and permissions
About this article
Cite this article
Herrero, P., Weinheimer, C.J., Dence, C. et al. Quantification of myocardial glucose utilization by pet and 1-carbon-11-glucose. J Nucl Cardiol 9, 5–14 (2002). https://doi.org/10.1067/mnc.2001.120635
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1067/mnc.2001.120635