Abstract
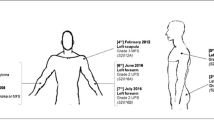
Glomus tumors are significantly rare tumors of carotid body. The great majority of these tumors are benign in character. Here we present two brothers with hereditary glomus jugulare tumor who had consanguineous parents. Radiotherapy was applied approximately 8 and 10 years ago for treatment in both cases. Eight years later, one of these cases came to our notice due to relapse. The mutation pattern of p53, p57KIP2, p16INK4A and p15NK4B genes which have roles in the cell cycle, was analyzed in tumor samples obtained from the two affected cases in the initial phase and from one of these cases at relapse. The DNA sample obtained from the case in initial diagnosis phase revealed no p53, p57KIP2, p16INK4A or p15INK4B mutation. He is still in remission phase. Despite the lack of p53, p57KIP2, p16INK4A and p15INK4B mutation at initial diagnosis the tumor DNA of the other case in relapse revealed p53 codon 243 (ATG→ATC; met→ile) and p16 codon 97 (GAC→AAC; asp→asn) missense point mutations. No loss of heterozygosity in p53 and p16INK4A was observed by microsatellite analysis of tumoral tissues in these cases. P53 and p16INK4A mutations observed in relapse phase were in conserved regions of both genes. No previous reports have been published with these mutations in glomus tumor during progression. The mutation observed in this case may due to radiotherapy. In spite of this possibility, the missense point mutations in conserved region of p53 and p16INK4A genes may indicate the role of p53 and p16INK4A in tumor progression of glomus tumors.
Similar content being viewed by others
References
Hirschfeld A, Kornblith P: Uncommon tumors of the nervous system. In Textbook of uncommon cancer. Eds: Williams CJ, Krikorian JG, Green MR, Raghavan D. New York John Wiley and Sons 1988, pp 590–612.
Baysal BE, von Schorthost FM, Farr JF, et al: A high resolution STS, FST and gene based physical map of the hereditary paraganglioma region on chromosome 11q23. Genomics 44:214–221, 1997.
Oosterwijk JC, Jansen JC, nvon Schothorst FM, et al: First experience with genetic counseling based on predictive DNA diagnosis in hereditary glomus tumorsparagangliomas. J Med Genet 33:379–383, 1996.
Wang CC: Paraganglioma of the head and neck, In Textbook of uncommon cancer Eds: Williams CJ, Krikorian JG, Green MR, Raghavan D. New York, John Wiley and Sons. 1988, pp 725–729.
Hiruta N, Kameda N, Takudome T, et al: Malignant glomus tumor: a case report and review of the literature. Am J. Surg Pathol 21:1096–1103, 1997.
Imamura J, Miyashi I, Koeffler PH: P53 in haematologic malignancies. Blood 84:2412–2421, 1994.
Marx J: How p53 suppress cell growth. Science 262:1644–1645, 1993.
el-Deiry DW, Harper JW, OConnor PM, et al: WAF1/ CIP 1 is induced in p 53-mediated G1 arrest and apopitosis. Cancer Res 54:1169–1174, 1994.
Morgan DO: Principles of CDK regulation. Nature 374:131–134, 1995.
Nurse P: Ordering S phase and M phase in the cell cycle. Cell 79:547–550, 1994.
Sherr CS: G1 phase progression: Cycling on clue. Cell 79:551–555, 1994.
Hunter T and Pines J: Cyclins and cancer II: Cyclin D and CDK inhibitors come of age. Cell 79:573–582, 1994.
Guan KL, Jenkins CW, Li Y, et al: Growth suppression by p18, p16(INK4/MTS1) and p14 (INK4B/MTS2)-related CDK6 inhibitor, correlates with wide-type pRb function. Genes Dev 8:2939–2952, 1994.
Lee MH, Reynisdottir I, Massague J: Cloning of p57KIP2, a cyclin dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev 9:639–649, 1995.
Matsouka S, Edwards MC, Bai C, et al: p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev 9:650–662, 1995.
Zhang Y, Xiong Y, Yarbrough WG: ARF promotes MDM2 degredation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell 92:725–734, 1998.
Pomerantz J, Schreiber-Agus N, Silverman A, et al: The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2’ s inhibition of p53. Cell 92:713–725, 1998.
Lo Coco F, Gaidano G, Louie DC, et al: P53 mutations are associated with histologic transformation of follicular lymphoma. Blood 82:2289–2295, 1993.
Güran Ş, Bahçe M, Beyan C, et al: During the progression of Chronic Myeloid Leukemia, p53, p15, p16 and p57 mutations. Haemotologia (in press).
Güran Ş, Pak I: Cumulation of TP53 mutations and p15p16 homozygous deletions in HPV type 16 positive scrotal cancers. Cancer Genet Cytogenet (in press).
Tali ET, Şener RN, IbiŜ E, et al: Familial bilateral glomus jugulare tumors, Neuroradiology 33:171–172, 1991.
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning. New York. Cold Spring Harbor Lab Press 1998.
Wright DK, Manos MM: Simple preparation from paraffinembedded tissues. In PCR Protocols, a guide to methods and applications. Eds. Innis MA. San Diego, Academic Press Inc. pp. 153–158 1990.
Liu L, Lassam NJ, Slingerland JM, et al: Germline p16 INK4A mutation and protein dysfunction in a family with inherited melanoma. Oncogene 11:405–412, 1995.
Buchman VL, Chumakov PM, Ninkina NN, et al: A variation in the structure of the protein-coding region of human p53 gene. Gene 70:245–252, 1988.
Lee PM, De Baun M, Randhava G, et al: Low frequency of p57KIP2 mutation in Beck with-Wiedemann Syndrome. Am J Hum Genet 61:304–309, 1997.
Orita M, Suzuki Y, Sekiya , et al: Rapid and sensitive detection of point mutations and DNA polymorphism using the polymerase chain reaction. Genomics 5:874–879, 1989.
Piqueras JF, Santos J, Castro IP, et al: Frequent allelic losses of 9p21 markers and low insidence of mutations at p16 (CDKN2) gene in non-Hodgkin’s lymphomas of B-cell lineage. Cancer Genet Cytogenet 98:63–68, 1987.
Weissenbach J, Gyapay G, Dib D, et al: A second-generation linkage map of the human genome. Nature 359, 798–801, 1992.
Liu Q, Neuhausen S, Mc Clure M, et al: CDKN2 (MST1) tumor suppressor gene mutations in human cancer cell lines. Oncogene 10:1061–1067, 1995.
Nobori T, Miura K, Wu DJ, et al: Deletions of the cyclin-dependent kinase -4 inhibitor gene in multiple human cancers. Nature 368, 753–756, 1994.
Kwiatkowski DJ, Diaz MO: Dinucleotide repeat polymorphism at the IFNA locus (9p22). Human Mol Genet 1:658, 1992.
Hahn M, Serth J, Fislage R, et al: Polymerase chain reaction detection of a highly polymorphic VNTR segment in intron 1 of the human p53 gene (letter). Clin Chem 39:549–550, 1993.
Mc Daniel T, Carbone D, Takahashi T, et al: The Msp I polymorphism in intron 6 of p53 (TP53) detected by digestion of PCR products. Nucleic Acids Res 19:4796, 1991.
Barel D, Avigad S, Mor C, et al: A novel germ-line mutation in the non-coding region of the p53 gene in a Li-Fraumeni family. Cancer Genet Cytogenet 103:1–6, 1998.
Kiyosawa T, Umebetashi Y, Nakayama Y, et al: Hereditary multiple glomus tumors involving the gleans penis. A case report and review of the literature. Dermatol Surg 21:895–899, 1995.
Brathwaite CD, Poppiti RS: Malignant glomus tumor. A case report of widespread metastases in a patient with multiple glomus body hamartomas. Am J Surg Pathol 20:233–238, 1996.
Sorensen BS, Hovic E: CDKN2A (P16INK4A) somatic and germline mutations. Human Mutation 7:294–303, 1996.
Alberts B, Bray D, Lewis J, et al: Cancer eds.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD in Molecular biology of the cell. New York. Garland Publishing. 1994 pp. 1255–1291.
Gabriel FM, Sampson JH, Dodd IG, et al: Glomus jugulare tumor metastatic to the sacrum after high-dose radiation therapy: a case report. Neurosurgery 37:1001–1005, 1995.
Levine AS, Momand S, Finlay AC: The p53 tumor suppressor gene. Nature 351:453–458, 1991.
Lilischikis R, Sorcevic R, Kennedy C, et al: Cancer-associated missense and deletion mutations impair p16 INK4 CDK inhibitory activity. Int J Cancer 66:294–304, 1996.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
GÜran, Ş., Tali, E.T. p53 and p16INK4A Mutations during the progression of glomus tumor. Pathol. Oncol. Res. 5, 41–45 (1999). https://doi.org/10.1053/paor.1999.0041
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1053/paor.1999.0041