Abstract
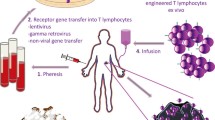
The purpose of this review is to illustrate some of the technical and biological hurdles that need to be addressed when developing new gene therapy based clinical trials. Gene transfer approaches can be used to “mark” cells to monitor their persistence in vivo in patients, to protect cells from toxic chemotherapeutic agents, correct a genetic defect within the target cell, or to confer a novel function on the target cell. Selection of the most suitable vector for gene transfer depends upon a number of factors such as the target cell itself and whether gene expression needs to be sustained or transient. The TCR gene transfer approach described here represents one innovative strategy being pursued as a potential therapy for metastatic melanoma. Tumor reactive T cells can be isolated from the tumor infiltrating lymphocytes (TIL) of melanoma patients. A retroviral vector has been constructed containing the T cell receptor (TCR) α and β chain genes from a MART-1(27-35)-specific T cell clone (TIL 5). Jurkat cells transduced with this virus specifically release cytokine in response to MART-1(27-35) peptide pulsed T2 cells, showing that the virus can mediate expression of a functional TCR. HLA-A2 transgenic mice are being used to examine whether transduced bone marrow progenitor cells will differentiatein vivo into mature CD8+ T cells expressing the MART-1(27-35)-specific TCR. Expression of the human TCR α and β chain genes has been detected by RT-PCR in the peripheral blood of HLA-A2 transgenic mice reconstituted with transduced mouse bone marrow. Expression of the TIL 5 TCR genes in the peripheral blood of these mice was maintained for greater than 40 weeks after bone marrow reconstitution. TIL 5 TCR gene expression was also maintained following transfer of bone marrow from mice previously reconstituted with transduced bone marrow to secondary mouse recipients, suggesting that a pluripotent progenitor or lymphocyte progenitor cell has been transduced.
Similar content being viewed by others
References
Rosenberg SA, Abersold P, Cornetta K, et al: Gene transfer into humans: immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med 323:570–578, 1990.
Blaese RM, Culver KW, Miller AD, et al: T lymphocyte-directed gene therapy for ADA-SCID: initial trial results after 4 years. Science 270:475–480, 1995.
Partridge TA, Davies KE: Myoblast-based gene therapies. Br Med Bull 51:123–137, 1995.
Alton EW, Middleton PG, Caplen NJ, et al: Non-invasive liposome-mediated gene delivery can correct the ion transport defect in cystic fibrosis mutant mice. Nat Genet 5:135–142, 1993.
Favrot M, Coll JL, Louis N, et al: Cell death and cancer: replacement of apoptotic genes and inactivation of death suppressor genes in therapy. Gene Ther 5:728–739, 1998.
Vandendriessche T, Chuah MK, Chiang L, et al: Inhibition of clinical human immunodeficiency virus (HIV) type I isolates in primary CD4+ T lymphocytes by retroviral vectors expressing anti-HIV genes. J Virol 69:4045–4052, 1995.
Pardoll D: Immunotherapy with cytokine gene-transduced tumor cells: the next wave in gene therapy for cancer. Curr Opin Oncol 4:1124–1129, 1992.
Licht T, Herrmann F, Gottesman MM, et al: In vivo drug-selectable genes: a new concept in gene therapy. Stem Cells 15:104–111, 1997.
Greenberg PD, Finch RJ, Gavin MA, et al: Genetic modification of T-cell clones for therapy of human viral and malignant diseases. The Cancer J from Scientific American 4 (suppl. 1): S100-S105, 1998.
Pogulis RJ, Hansen MJ, Pease LR: Retroviral-mediated expression of an MHC class I-restricted T cell receptor in the CD8 T cell compartment of bone marrow-reconstituted mice. Human Gene Ther 9:2285–2297, 1998.
Brenner MK: The contribution of marker gene studies to hemopoietic stem cell therapies. Stem Cells 13:453–461, 1995.
Kessler PD, Podasakoff GM, Chen X, et al: Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA 93:14082–14087, 1996.
Fisher KJ, Jooss K, Alston J, et al: Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med 3:306–312, 1997.
Monahan PE, Samulski RJ, Tazelaar J, et al: Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia. Gene Ther 5:40–49, 1998.
Yang Y, Li Q, Ertl HC, et al: Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol 69:2004–2015, 1995.
Miller AD, Rosman GJ: Improved retroviral vectors for gene transfer and expression. Biotechniques 7:980–990, 1989.
Treisman J, Hwu P, Minamoto S, et al: Interleukin-2-transduced lymphocytes grow in an autocrine fashion and remain responsive to antigen. Blood 85:139–145, 1995.
Markowitz D, Goff S, Bank A: A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol 62:1120–1124, 1988.
Miller AD, Garcia JV, von Suhr N, et al: Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol 65:2220–2224, 1991.
Miller AD, Buttimore C: Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Molec Cell Biol 6:2895–2902, 1986.
Pockaj BA, Sherry RM, Wei JP, et al: Localization of111Indiumlabelled tumor infiltrating lymphocytes to tumor in patients receiving adoptive immunotherapy. Augmentation with cyclo-phosphamide and correlation with response. Cancer 15:1731–1737, 1994.
Kast WM, Offringa R, Peters PJ, et al: Eradication of adenovirus E1-induced tumors by E1A-specific cytotoxic T lymphocytes. Cell 59:603–614, 1989.
Rodolfo M, Bassi C, Salvi C, et al: Therapeutic use of a long-term T cell line recognizing a common tumor associated antigen: the pattern of in vitro reactivity predicts the in vivo effect on different tumors. Cancer Immunol Immunother 34:53–63 1991.
Feltkamp MC, Vreugenhil GR, Vierboom MPM, et al: Cytotoxic T lymphocytes raised against a subdominant epitope offered as a synthetic peptide eradicate human papillomavirus type 16-induced tumors. Eur J Immunol 25:2638–2642, 1995.
Burger UL, Chang MP, Nagoshi M, et al: Improved in vivo efficiency of tumor-infiltrating lymphocytes after restimulation with irradiated tumor cells in vitro. Ann Surg Oncol 3:580–587, 1996.
Shilyansky J, Yang JC, Custer MC, et al: Identification of a T-cell receptor from a therapeutic murine T-cell clone. J Immunother Emphasis Tumor Immunol 20:247–255, 1997.
Rosenberg SA, Yannelli JR, Yang JC, et al: Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst 86:1159–1166, 1994.
Kawakami Y, Eliyahu S, Sakaguchi K, et al: Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med 180:347–352, 1994.
Kawakami Y, Eliyahu S, Delgado CH, et al: Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA 91:3515–3519, 1994.
Cole DJ, Weil J, Shilyansky J, et al: Characterization of the functional specificity of a cloned T-cell receptor heterodimer recognizing the MART-1 melanoma antigen. Cancer Res 55:748–752, 1995.
Kawasaki ES: In: PCR Protocols (eds. Innis, MA, Gelfand, DH, Sninsky, JJ and White, TJ) 146–152 (Academic Press, San Diego, CA, 1990).
Orkin SH, Motulsky AG: Report and recommendations of the panel to assess the NIH investment in research on gene therapy. NIH panel report. 1996.
Blaese RM, Anderson WF: The ADA human gene therapy clinical protocol. Human Gene Ther 1:327–362, 1990.
Blaese RM, Anderson WF, Culver K: The ADA human gene therapy clinical protocol. Human Gene Ther 4:521–527, 1990.
Rosenberg SA, Kasid A, Anderson WF, et al: TNF/TIL human gene therapy clinical protocol. Human Gene Ther 1:441–480, 1990.
Rosenberg SA, Anderson WF, Blaese MR, et al: Immunization of cancer patients using autologous cancer cells modified by insertion of the gene for interleukin-2. Human Gene Ther 3:75–91, 1992.
Cassileth PA, Podack E, Sridhar K, et al: Phase I study of transfected cancer cells expressing the interleukin-2 gene product in limited stage small cell lung cancer. Human Gene Ther 6:369–383, 1995.
Das Gupta TK, Cohen EP and Richards JM: Phase I evaluation of interleukin-2-transfected irradiated allogeneic melanoma for the treatment of metastatic melanoma, Human Gene Ther 8:1713–1726, 1997.
Department of Health and Human Services, National Institutes of Health Recombinant DNA Advisory Committee, minutes of meeting, June 9-10, 1994. Human Gene Ther 6:249–252, 1995.
Department of Health and Human Services, National Institutes of Health Recombinant DNA Advisory Committee, minutes of meeting, June 9-10, 1994. Human Gene Ther 6:255, 1995.
Department of Health and Human Services, National Institutes of Health Recombinant DNA Advisory Committee, minutes of meeting, September 12-13, 1994. Human Gene Ther 6:498–499, 1995.
Department of Health and Human Services, National Institutes of Health Recombinant DNA Advisory Committee, minutes of meeting, March 6-7, 1995. Human Gene Ther 6:1634–1636, 1995.
Department of Health and Human Services, National Institutes of Health Recombinant DNA Advisory Committee, minutes of meeting, June 8-9, 1995. Human Gene Ther 7:432–434, 1996.
Department of Health and Human Services, National Institutes of Health Recombinant DNA Advisory Committee, minutes of meeting, June 8-9, 1995. Human Gene Ther 7:432, 1996.
Rosenberg SA, Anderson WF, Asher AL, et al: Immunization of cancer patients using autologous cancer cells modified by insertion of the gene for tumor necrosis factor. Human Gene Ther 3:57–73, 1992.
Berns AJM, Clift S, Cohen LK, et al: Phase I study of non-replicating autologous tumor cell injections using cells prepared with or without GM-CSF gene transduction in patients with metastaic renal cell carcinoma. Human Gene Ther 6:347–368, 1995.
Seigler HF, Darrow TL, Abdel-Wahab Z, et al: A phase I trial of human gamma interferon transduced autologous tumor cells in patients with disseminated malignant melanoma. Human Gene Ther 5:761–773, 1994.
Dranoff G, Soiffer R, Lynch T, et al: A phase I study of vaccination with autologous, irradiated melanoma cells engineered to secrete human granulocyte-macrophage colony stimulating factor. Human Gene Ther 8:111–123, 1997.
Department of Health and Human Services, National Institutes of Health Recombinant DNA Advisory Committee, minutes of meeting, March 6-7, 1995. Human Gene Ther 6:1631–1634, 1995.
Department of Health and Human Services, National Institutes of Health Recombinant DNA Advisory Committee, minutes of meeting, September 11-12. Human Gene Ther 7:1033, 1996.
Lotze MT, Rubin JT, Carty S, et al: Gene therapy of cancer: a pilot study of IL-4-gene-modified fibroblasts admixed with autologous tumor to elicit an immune response. Human Gene Ther 5:41–55, 1994.
Sobol RE, Royston I, Fakhrai H, et al: Injection of colon carcinoma patients with autologous irradiated tumor cells and fibroblasts genetically modified to secrete interleukin-2 (IL-2): a phase I study. Human Gene Ther 6:195–204, 1995.
Tahara H, Lotze MT, Robbins PD, et al: IL-12 gene therapy using direct injection of tumors with genetically engineered autologous fibroblasts. Human Gene Ther 6:1607–1624, 1995.
Deisseroth AB, Kavanagh J, Champlin R: Use of safety-modified retroviruses to introduce chemotherapy resistance sequences into normal hematopoietic cells for chemoprotection during the therapy of ovarian cancer: a pilot trial. Human Gene Ther 5:1507–1522, 1994.
Hesdorffer C, Antman K, Bank A, et al: Human MDR gene transfer in patients with advanced cancer. Human Gene Ther 5:1151–1160, 1994.
OShaughnessy JA, Cowan KH, Nienhuis AW,et al: Retroviral mediated transfer of the human multidrug resistance gene (MDR-1) into hematopoietic stem cells during autologous transplantation after intensive chemotherapy for metastatic breast cancer. Human Gene Ther. 5:891–911, 1994.
Deisseroth AB, Holmes F, Hortobagyi G, et al: Use of safety-modified retroviruses to introduce chemotherapy resistance sequences into normal hematopoietic cells for chemoprotection during the therapy of breast cancer; a pilot trial. Human Gene Ther7:401–416, 1996.
Department of Health and Human Services, National Institutes of Health Recombinant DNA Advisory Committee, minutes of meeting, June 7-8, 1993. Human Gene Ther 5:537–539, 1994.
Department of Health and Human Services, National Institutes of Health Recombinant DNA Advisory Committee, minutes of meeting, September 11-12, 1995. Human Gene Ther 5:1036–1038, 1996.
Holt JA, Arteaga CB, Robertson D, et al: Gene therapy for the treatment of metastatic breast cancer by in vivo transduction with breast-targeted retroviral vector expressing antisense c-fos RNA. Human Gene Ther 7:1369–1380, 1996.
Roth JA: Modification of tumor supressor gene expression in non-small cell lung cancer (NSCLC) with a retroviral vector expressing wildtype (normal) p53. Human Gene Ther 7:861–874, 1996.
Roth JA: Modification of tumor supressor gene expression and induction of apoptosis in non-small cell lung cancer (NSCLC) with an adenovirus vector expressing wildtype p53 and cisplatin. Human Gene Ther 7:1013–1030, 1996.
Department of Health and Human Services, National Institutes of Health Recombinant DNA Advisory Committee, minutes of meeting, June 12-13, 1997. Human Gene Ther 9:394–402, 1998.
Culver KW, van Gilder J, Link CJ, et al: Gene therapy for the treatment of malignant brain tumors with in vivo tumor transduction with the Herpes Simplex thymidine kinase gene/ganciclovir system. Human Gene Ther 5:343–379, 1994.
Raffel C, Culver KW, Kohn D, et al: Gene therapy for the treatment of recurrent pediatric malignant astrocytomas with in vivo tumor transduction with the Herpes Simplex thymidine kinase gene/ganciclovir system. Human Gene Ther 5:863–890, 1994.
Galpin JE, Casciato DA, Richards SB: A phase I clinical trial to evaluate the safety and biological activity of HIV-IT(TAF)(HIV-1IIIBenv-transduced, autologous fibroblasts) in asymptomatic HIV-1 infected subjects. Human Gene Ther 5:997–1017, 1994.
Nabel GJ, Fox BA, Post L, et al: A molecular genetic intervention for AIDS - effects of a transdominant negative form of Rev. Human Gene Ther 5:79–92, 1994.
Haubrich R, McCutchan JA: An open label, phase I/II clinical trial to evaluate the safety and biological activity of HIV-IT(V)(HIV-1IIIB env/env retroviral vector) in HIV-1-infected subjects. Human Gene Ther 6:941–959, 1995.
Department of Health and Human Services, National Institutes of Health Recombinant DNA Advisory Committee, minutes of meeting, September 9-10, 1993. Human Gene Ther 5:655–657, 1994.
Morgan R and Walker,R: Gene therapy for AIDS using retroviral mediated gene transfer to deliver HIV-1 antisense TAR and transdominant Rev protein genes to syngeneic lymphocytes in HIV-1 infected identical twins. Human Gene Ther 7:1281–1306, 1996.
Crystal RG, Jaffe A, Brody S, et al: A phase I study, in cystic fibrosis patients, of the safety, toxicity, and biological efficacy of a single administration of a replication deficient, recombinant adenovirus carrying the cDNA of the normal cystic fibrosis transmembrane conductance regulator gene in the lung. Human Gene Ther 6:643–666, 1995.
Wilson JM, Engelhardt JF, Grossman M, et al: Gene therapy of cystic fibrosis lung disease using E1 deleted adenoviruses: a phase I trial. Human Gene Ther 5:501–519, 1994.
Welsh MJ, Smith AE, Zabner J, et al: Cystic fibrosis gene therapy using an adenovirus vector: in vivo safety and efficacy in nasal epithelium. Human Gene Ther 5:209–219, 1994.
Wilmott RW, Whitsett JA, Trapnell B, et al: Gene therapy for cystic fibrosis utilizing a replication deficient recombinant adenovirus vector to deliver the human cystic fibrosis transmembrane conductance regulator cDNA to the airways. A phase I study. Human Gene Ther 5:1019–1057, 1994.
Boucher RC, Knowles MR, Johnson LG, et al: Gene therapy for cystic fibrosis using E1-deleted adenovirus: a phase I trial in the nasal cavity, the University of North Carolina. Human Gene Ther 5:615–639, 1994.
Sorcher EJ, Logan JJ, Frizzell RA, et al: Gene therapy for cystic fibrosis using cationic liposome mediated gene transfer: a phase I trial of safety and efficacy in the nasal airway. Human Gene Ther 5:1261–1279, 1994.
Welsh MJ, Zabner J, Graham SM, et al: Adenovirus-mediated gene transfer for cystic fibrosis: Part A. Safety of dose and repeat administration in the nasal epithelium. Part B. Clinical efficacy in the maxillary sinus. Human Gene Ther. 6:205–218, 1995.
Flotte TR, Carter B, Conrad C, et al: A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Human Gene Ther 7:1145–1159, 1996.
Crystal RG, Mastrangeli A, Sanders A, et al: Evaluation of repeat administration of a replication deficient, recombinant adenovirus containing the normal cystic fibrosis transmembrane conductance regulator cDNA to the airways of individuals with cystic fibrosis. Human Gene Ther 6:667–703, 1995.
Dunbar C, Kohn D, Karlsson S, et al: Retroviral mediated transfer of the cDNA for human glucocerebrosidase into hematopoietic stem cells of patients with Gaucher disease. A phase I study. Human Gene Ther 7:231–253, 1996.
Department of Health and Human Services, National Institutes of Health Recombinant DNA Advisory Committee, minutes of meeting, December 2-3, 1993. Human Gene Ther 5:1178–1180, 1994.
Department of Health and Human Services, National Institutes of Health Recombinant DNA Advisory Committee, minutes of meeting, June 9-10, 1994. Human Gene Ther 6:246–247, 1995.
Department of Health and Human Services, National Institutes of Health Recombinant DNA Advisory Committee, minutes of meeting, September 12, 1997. Human Gene Ther 9, 914, 1998.
Whitley CB, McIvor RS, Aronovich EL, et al: Retroviral-mediated transfer of the Iduronate-2-sulfatase gene into lymphocytes for treatment of mild Hunter syndrome (Mucopolysaccharidosis Type II). Human Gene Ther 7:537–549, 1996.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clay, T.M., Custer, M.C., Spiess, P.J. et al. Potential use of T cell receptor genes to modify hematopoietic stem cells for the gene therapy of cancer. Pathol. Oncol. Res. 5, 3–15 (1999). https://doi.org/10.1053/paor.1999.0003
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1053/paor.1999.0003