Abstract
-
• The development of truffles in the soil is not well understood. It is not known if a direct transfer of carbohydrates takes place between the host tree and the developing ascocarps through ectomycorrhizal structures or whether sporophores become independent from their hosts after several weeks or months and are able to use dead host tissues or soil organic matter as carbon (C) and nitrogen (N) sources.
-
• To study saprophytic or symbiotic capacities of truffle ascocarps the natural abundance of 15N and 13C in foliage, wood, fine roots, mycorrhizae, fungal sporophores and soil were determined in a truffle orchard.
-
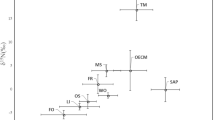
• The processes of carbon and nitrogen allocation remained unchanged during the entire period of ascocarp development of Tuber melanosporum. From 13C and 15N natural abundance measurements, T. melanosporum, T. brumale and T. rufum did not exhibit saprotophic strategy during ascocarp development, which is contradictory to common statements found in handbooks regarding truffle cultivation.
Résumé
-
• Le développement des truffes dans le sol n’est pas encore bien compris. Les connaissances actuelles ne nous permettent pas de savoir s’il existe un transfert direct de sucres entre l’arbre hôte et les ascocarpes en développement via les structures ectomycorhiziennes, ou si les ascocarpes utilisent le carbone et l’azote directement issu de la matière organique du sol.
-
• Nous avons mesuré l’abondance naturelle du 15N et du 13C dans le sol, les feuilles, les mycorhizes, le bois et les carpophores d’une truffière naturelle à chêne vert afin de déterminer la stratégie de la nutrition carbonée des ascocarpes.
-
• Les processus d’allocation du carbone et de l’azote restent identiques pendant toute la phase de développement des ascocarpes de Tuber melanosporum. De ces mesures d’abondance naturelle du 15N et du 13C, il apparaît que T. melanosporum, T. brumale et T. rufum ne développent pas de stratégie saprophytique pendant le développement des ascocarpes, ce qui est en contradiction avec les idées habituellement véhiculées par les manuels de trufficulture
Similar content being viewed by others
References
Anne P., 1945. Sur le dosage rapide du carbone organique des sols, Ann. Agron. 2: 161–172.
Barry D., 1992. Croissance et fonctionnement d’un ascocarpe au stade adulte de Tuber melanosporum et Tuber aestivum. Étude structurale des hyphes externes et approche expérimentale de leur fonction. Thèse de Doctorat en Sciences Agronomiques, ENSAM Montpellier, 155 p.
Barry D., Callot G., Janex-Favre M.C., Pargney J.C., and Parguey-Leduc A., 1993. Morphologie des hyphes externes observées sur le péridium des Tuber à écailles: évolution au cours du développement de l’ascocarpe. Can. J. Bot. 71: 609–619.
Barry D., Staunton S., and Callot G., 1994. Mode of the absorption of water and nutrients by ascocarps of Tuber melanosporum and Tuber aestivum. A radioactive tracer technique. Can. J. Bot. 72: 317–322.
Bidartondo M.I., Burghardt B., Gebauer G., Bruns T., and Read D., 2004. Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proc. R. Soc. 271: 1799–1806.
Bremmer J.M., 1960. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 55: 11–33.
Callot G. and Guyon A., 1990. Microstructural analysis of the truffle ascocarp interface during development. Proc. of 14th Int. Congress of Soil Science, Kyoto, Japan, pp. 256–261.
Callot G., Bye P., Raymond M., Fernandez D., Pargney J.C., Parguey-Leduc A., Janex-Favre M.C., Moussa R., and Pages L., 1999. La truffe, la terre, la vie. Éditions INRA, Paris, 210 p.
Ceccaroli P., Saltarelli R., Cesari P., Pierleoni R., Saccocini C., Vallorani L., Stocchi V., and Martin F., 2003. Carbohydrate and amino acid metabolism in Tuber borchii mycelium during glucose utilization: a 13C NMR study. Fungal Genet. Biol. 39: 168–179.
Courty P.E., Pritsch K., Schloter M., Hartmann A., and Garbaye J., 2005. Activity profiling of ectomycorrhiza communities in two forest soils using multiple enzymatic tests. New Phytol. 167: 309–319.
Courtecuisse R., 2000. Mushrooms of Britain and Europe, Harper Collins, 904 p.
Duchaufour P. and Bonneau M., 1959. Une nouvelle méthode de dosage du phosphore assimilable dans les sols forestiers. Bulletin de l’Association Française d’Étude des Sols 4: 193–198.
Emmerton K.S., Callaghan T.V., Jones H.E., Leake J.R., Michelsen A., and Read D.J., 2001a. Assimilation and isotopic fractionation of nitrogen by mycorrhizal fungi. New Phytol. 151: 503–511
Emmerton K.S., Callaghan T.V., Jones H.E., Leake J.R., Michelsen A., and Read D.J., 2001b. Assimilation and isotopic fractionation of nitrogen by mycorrhizal and nonmycorrhizal subarctic plants. New Phytol. 151: 513–524.
France R.C. and Reid C.P.P., 1983. Interactions of nitrogen and carbon in the physiology of ectomycorrhizae. Can. J. Bot. 61: 964–984.
Gardes M. and Bruns T.D., 1993. ITS primers with enhanced specificity for basidiomycetes — application to the identification of mycorrhizae and rusts. Mol. Ecol. 2: 113–118.
Gebauer G. and Dietrich P., 1993. Nitrogen isotope ratios in different compartments of a mixed stand of spruce, larch and beech trees and of understorey vegetation including fungi. Isotopenpraxis 29: 35–44.
Gebauer G. and Taylor A.F.S., 1999. 15N natural abundance in fruit bodies of different functional groups of fungi in relation to substrate utilization. New Phytol. 142: 93–101.
Gebauer G. and Meyer M., 2003. 15N and 13C natural abundance of autotrophic and myco-heterotrophic orchids provides insight into nitrogen and carbon gain from fungal association. New Phytol. 160: 209–223.
Henn M.R. and Chapela I.H., 2001. Ecophysiology of 13C and 15N isotopic fractionation in forest fungi and the roots of the saprotrophicmycorrhizal divide. Oecologia 128: 480–487.
Hobbie E.A., 2005 Using isotopic tracers to follow carbon and nitrogen cycling in fungi. In: Dighton J., White J. and Oudemans P. (Eds.), The Fungal Community: Its Organization and Role in the Ecosystem, 3rted CRC Press, pp. 361–381.
Hobbie E.A. and Colpaert J.V., 2003. Nitrogen availability and colonization by mycorrhizal fungi correlate with nitrogen isotope patterns in plants. New Phytol. 157: 115–126.
Hobbie E.A. and Colpaert J.V., 2004. Nitrogen availability and mycorrhizal colonization influence water use efficiency and carbon isotope patterns in Pinus sylvestris. New Phytol. 164: 515–525.
Hobbie E.A., Macko S.A., and Shugart H., 1999. Insights into nitrogen and carbon dynamics of ectomycorrhizal and saprotrophic fungi from isotopic evidence. Oecologia 118: 353–360.
Hobbie E.A., Weber N.S., and Trappe J.M., 2001. Mycorrhizal vs. saprotrophic status of fungi: the isotopic evidence. New Phytol. 150: 601–610.
Hobbie E.A., Weber N.S., Trappe J.M., and van Klinken G.J., 2002. Using radiocarbon to determine the mycorrhizal status of fungi. New Phytol. 156: 129–136.
Hobbie E.A., Sanchez F.S., and Rygiewicz P.T., 2004. Carbon use, nitrogen use, and isotopic fractionation of ectomycorrhizal and saprotrophic fungi in natural abundance and 13C-labelled cultures. Mycol. Res. 108: 725–736.
Hobbie E.A., Jumpponen A., and Trappe J.M., 2005. Foliar and fungal 15N:14N ratios reflect development of mycorrhizae and nitrogen supply during primary succession: testing analytical models. Oecologia 146: 258–268.
Högberg P., 1997. 15N natural abundance in soil-plant systems. New Phytol. 137: 179–203.
Högberg P., Högbom L., Schinkel H., Högberg M., Johannisson C., and Wallmark H., 1996. 15N abundance of surface soils, roots and mycorrhizae in profiles of European forest soils. Oecologia 108: 207–214.
Kohzu A., Yoshioka T., Ando T., Takahashi M., Koba K., and Wada E., 1999. Natural 13C and 15N abundance of field-collected fungi and their ecological implications. New Phytol. 144: 323–330.
Kohzu A., Tateishi T., Yamada A., Koba K., and Wada E., 2000. Nitrogen isotope fractionation during nitrogen transport from ectomycorrhizal fungi, Suillus granulatus, to the host plant, Pinus densiflora. J. Soil Sci. Plant Nutr. 46: 733–739.
Lacourt I., Duplessis S., Abbà S., Bonfante P., and Martin F., 2002. Isolation and characterization of differentially expressed genes in the mycelium and fruit body of Tuber borchii. Appl. Environ. Microbiol. 68: 4574–4582.
Lapeyrie F., 2002. Oxalate synthesis from soil bicarbonate by the mycorrhizal fungus Paxillus involutus. Plant Soil 110: 3–8.
Leake J.T., 2001. Is diversity of ectomycorrhizal fungi important for ecosystem function? New Phytol. 152: 1–3.
Le Tacon F., Delmas J., Gleyze R., and Bouchard D., 1982. Influence du régime hydrique du sol et de la fertilisation sur la fructification de la truffe noire du Périgord (Tuber melanosporum Vitt.) dans le sud-est de la France. Acta Oecol. 4: 291–306.
Lilleskov E.A., Hobbie E.A., and Fahey T.J., 2002. Ectomycorrhizal fungal taxa differing in response to nitrogen deposition also differ in pure culture organic nitrogen use and natural abundance of nitrogen isotopes. New Phytol. 154: 219–231.
Lindahl B., Stenlid J., Olsson S., and Finlay R., 1999. Translocation of 32P between interacting mycelia of a wood decomposing fungus and ectomycorrhizal fungi in microcosm systems. New Phytol. 144: 183–193.
Mamoun M. and Olivier J.M., 1991. Influence du substrat carboné et de la forme d’azote minéral sur la croissance de T. melanosporum Vittad. en culture pure. Application à la production de biomasse mycélienne. Agronomie 11: 521–527.
Marx A., de Graaf A.A., Wiechert W., Aggeling L., and Sahm H., 2000. Determination of the fluxes in the central metabolism of Corynebacterium glutamicum by nuclear magnetic resonance spectroscopy combined with metabolite balancing. Biotechnœ. Bioeng. 4: 111–129.
Olivier J.M., Savignac J.C., and Sourzat P., 1996. Truffe et trufficulture. Éditions Fanlac, Périgueux, France, 270 p.
Paolocci F., Rubini A., Riccioni C., and Arcioni S., 2006. Re-evaluation of the life cycle of Tuber magnatum. Appl. Environ. Microbiol. 72: 2390–2393.
Perez-Moreno J. and Read D.J., 2000. Mobilization and transfer of nutrients from litter to tree seedlings via the vegetative mycelium of ectomycorrhizal plants. New Phytol. 145: 301–309.
Pradel L., 1914. Manuel de trufficulture, guide pratique. Librairie JB Baillère et Fils, Paris, 1914.
Read D.J. and Perez-Moreno J., 2003. Mycorrhizas and nutrient cycling in ecosystems — a journey towards relevance? New Phytol. 157: 475–492.
Rommel L.G., 1938. A trenching experiment in spruce forest and its bearing on problems of mycotrophy. Sven. Bot. Tidsk. 32: 89–99.
Rouquerolle T. and Payre H., 1975. Conséquences de quelques particularités biologiques des Tuber sur les caractères des cultures de mycélium et sur la formation des truffes. Rev. Mycol. 29: 213–224.
Rouiller J., Guillet B., and Bruckert S., 1980. Cations acides échangeables et acidités de surface. Approche analytique et incidence pédogénétique, Bull. Assoc. Française d’Étude des Sols 2: 161–175.
Staaf H., 1988. Litter decomposition in beech forests — effects of excluding tree roots. Biol. Fertil. Soils 6: 302–305.
Straatsma G. and Bruinsma J., 1986. Carboxylated metabolic intermediates as nutritional factors in vegetative growth of the mycorrhizal mushroom Cantharellus cibarius Fr. J. Plant Physiol. 125: 377–381.
Taylor A.F.S., Fransson P.M., Högberg P., Högberg M.N., and Plamboeck A.H., 2003. Species level patterns in 13C and 15N abundance of ectomycorrhizal and saprotrophic fungal sporocarps. New Phytol. 159: 757–774.
Trudell S.A., Rygiewicz P.T., and Edmonds R.L., 2004. Patterns of nitrogen and carbon stable isotope ratios in macrofungi, plants and soils in two old-growth conifer forests. New Phytol. 164: 317–335.
White T.J., Bruns T., Lee S., and Taylor J., 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Gelfand M.A., Sninsky D.H., and White T.J. (Eds.), PCR Protocols: A guide to methods and applications, Academic Press, San Diego, CA, pp. 315–322.
Zeller B., Bréchet C., Maurice J.P., and Le Tacon F., 2007. 13C and 15N isotopic fractionation in trees, soils and fungi in a natural forest stand and a Norway spruce plantation. Ann. For. Sci. 64: 419–429.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeller, B., Bréchet, C., Maurice, JP. et al. Saprotrophic versus symbiotic strategy during truffle ascocarp development under holm oak. A response based on 13C and 15N natural abundance. Ann. For. Sci. 65, 607 (2008). https://doi.org/10.1051/forest:2008037
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1051/forest:2008037