Abstract
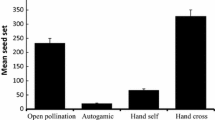
Plant population size has been shown to affect insect visitation and reproductive success. Small populations are at risk because individuals are more likely to be affected by stochastic processes and inbreeding depression (Allee effect). Additionally, several studies have found that plants in small populations also experience lower pollinator visitation rates, which may further decrease reproduction. In this study, seed set, pollinator visitation and pollen limitation of Thymus capitatus (L.) was assessed in 32 patches in eight populations of various sizes on the island of Lesvos, Greece. All populations except one were significantly pollen-limited. We found that although free-pollinated flowers produced more seeds in larger populations this was not due to higher pollinator visitation rates as flowers which received pollen supplements also produced more seeds in larger populations. We hypothesize that the higher seed set is due to a generally greater genetic variability or better habitat quality. We show that honeybee visitation alone significantly decreases pollen limitation.
Similar content being viewed by others
References
Ågren J. (1996) Population Size, pollinator limitation, and seed set in the self-incompatible herb Lythrum Salicaria, Ecol. Lett. 77, 1779–1790.
Amarasekare P. (2004) Spatial dynamics of mutualistic interactions, J. Anim. Ecol. 73, 128–142.
Blamey M., Grey-Wilson C. (2004) Wild Flowers of the Mediterranean, A & C Black, London.
Brys R., Jacquemyn H., De Bruyn L., Hermy M. (2007) Pollination success and reproductive output in experimental populations of the self-incompatible Primula vulgaris, Int. J. Plant Sci. 168, 571–578.
Brys R., Jacquemyn H., Endels P., Hermy M., De Blust G. (2003) The relationship between reproductive success and demographic structure in remnant populations of Primula veris, Acta Oecol. 24, 247–253.
Campbell D., Halama J. (1993) Resource and pollen limitation to lifetime seed production in a natural plant population, Ecology 74, 1043–1051.
Conner J.K., Neumeier R. (1995) Effects of black mustard population size on the taxonomic composition of pollinators, Oecologia 104, 218–224.
Fox L.R. (2007) Climatic and biotic stochasticity: disparate causes of convergent demographies in rare, sympatric plants, Conserv. Biol. 21, 1556–1561.
Ghazoul J. (2005) Pollen and seed dispersal among dispersed plants, Biol. Rev. 80, 413–443.
Henle K., Lindenmayer D.B., Margules C.R., Saunders D.A., Wissel C. (2004) Species survival in fragmented landscapes: where are we now? Biodivers. Conserv. 13, 1–8.
Iwaizumi M.G., Sakai S. (2004) Variation in flower biomass among nearby populations of Impatiens textori (Balsaminaceae): effects of population plant densities, Can. J. Bot. 82, 563–572.
Jennersten O., Nilsson S.G. (1993) Insect flower visitation frequency and seed production in relation to patch size of Viscaria vulgaris (Caryophyllaceae), Oïkos 68, 283–292.
Klein A.M., Steffan-Dewenter I., Tscharntke T. (2003a) Fruit set of highland coffee increases with the diversity of pollinating bees, Proc. R. Soc. Lond. B Biol. Sci. 270, 955–961.
Klein A.M., Steffan-Dewenter I., Tscharntke T. (2003b) Pollination of Coffea canephora in relation to local and regional agroforestry management, J. Appl. Ecol. 40, 837–845.
Klinkhamer P.G.L., van der Lugt P.-P. (2004) Pollinator service only depends on nectar production rates in sparse populations, Oecologia 140, 491–494.
Leimu R., Mutikainen P., Koricheva J., Fischer M. (2006) How general are positive relationships between plant population size, fitness and genetic variation? J. Ecol. 94, 942–952.
Michener C.D. (2000) The Bees of the World, Johns Hopkins University Press, Baltimore, USA.
Mustajärvi K., Siikamaki P., Rytkonen S., Lammi A. (2001) Consequences of plant population size and density for plant-pollinator interactions and plant performance, J. Ecol. 89, 80–87.
Paschke M., Abs C., Schmid B. (2002) Relationship between population size, allozyme variation, and plant performance in the narrow endemic Cochlearia bavarica, Conserv. Genet. 3, 131–144.
Petanidou T., Smets E. (1995) The potential of marginal lands for apiculture: nectar secretion in Mediterranean shrublands, Apidologie 26, 39–52.
Petanidou T., Smets E. (1996) Does temperature stress induce nectar production in Mediterranean plants? New Phytol. 133, 513–518.
Petanidou T., Vokou D. (1993) Pollination ecology of Labiatae in a phryganic (East Mediterranean) ecosystem, Am. J. Bot. 80, 892–899.
Rathcke B. (1983) Competition and facilitation among plants for pollination, Pollination Biol. 305–329.
Schemske D.W., Husband B.C., Ruckelshaus M.H., Goodwillie C., Parker I.M., Bishop J.G. (1994) Evaluating approaches to the conservation of rare and endangered plants, Ecology 75, 584–606.
Severns P. (2003) Inbreeding and small population size reduce seed set in a threatened and fragmented plant species, Lupinus sulphureus ssp. kincaidii (Fabaceae), Biol. Conserv. 110, 221–229.
Spira T.P. (2001) Plant-pollinator interactions: A threatened mutualism with implications for the ecology and management of rare plants, Nat. Areas J. 21, 78–88.
Steffan-Dewenter I., Tscharntke T. (2000) Resource overlap and possible competition between honey bees and wild bees in central Europe, Oecologia 122, 288–296.
Vamosi J.C., Knight T.M., Steets J.A., Mazer S.J., Burd M., Ashman T.L. (2006) Pollination decays in biodiversity hotspots, Proc. Natl. Acad. Sci. USA 103, 956–961.
Visscher P.K., Seeley T.D. (1982) Foraging strategy of honeybee colonies in a temperate deciduous forest, Ecology 63, 1790–1801.
Walther-Hellwig K., Fokul G., Frankl R., Buchler R., Ekschmitt K., Wolters V. (2006) Increased density of honeybee colonies affects foraging bumblebees, Apidologie 37, 517–532.
Westerkamp C. (1991) Honeybees are poor pollinators — why? Plant Syst. Evol. 177, 71–75.
Wolf A.T., Harrison S.P. (2001) Effects of habitat size and patch isolation on reproductive success of the serpentine morning glory, Conserv. Biol. 15, 111–121.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript editor: Stan Schneider
Rights and permissions
About this article
Cite this article
Tscheulin, T., Petanidou, T. Does spatial population structure affect seed set in pollen-limited Thymus capitatus?. Apidologie 42, 67–77 (2011). https://doi.org/10.1051/apido/2010035
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1051/apido/2010035