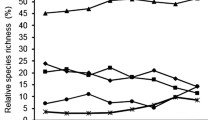
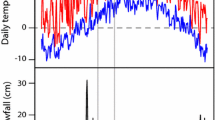
In riparian forest, insectivorous birds are largely dependent on aquatic preys. However, the contribution made by aquatic preys to bird diets varies considerably among bird species. In the present study, bird foraging behaviors were observed in order to examine the relationship between bird foraging method and the variation in the contribution of aquatic preys. The great tit, the black-faced bunting, and the wren are largely dependent on aquatic preys by capturing them on the ground. Sallyers, the brown flycatcher, the pale-legged willow warbler, and the narcissus flycatcher are also largely dependent on aquatic preys and capture them mostly during flight. The narcissus flycatcher frequently utilises aquatic invertebrates dwelling on the ground. The leaf and branch gleaner, the crowned willow warbler, does not depend on aquatic prey as much. Although both the pygmy woodpecker and nuthatch are branch and trunk gleaners, the nuthatch utilises aquatic preys frequently by capturing them on the ground as well as during flight, but the pygmy woodpecker does not depend on aquatic preys. The marsh tit also does not depend on aquatic preys but carefully searches terrestrial prey that hide in the vegetation. The differential dependence on aquatic preys among species can lead to the heterogeneous distribution of birds within a riparian forest, suggesting that the indirect effect of aquatic preys on a forest ecosystem via birds can vary within a forest–stream ecotone.
Similar content being viewed by others
References
Ter Braak C. J. F. & Smilauer P. (1998) Canoco Reference Manual and User’s Guide to Canoco for Windows. Software for Canonical Community Ordination (Version 4). Center for Biometry Wageningenm CPRO-DLO, Wageningenm, The Netherlands.
DeAngelis D. L. (1980) Energy flow, nutrient cycling, and ecosystem resilience. Ecology 61: 764–777.
Fagan W. F., Cantrell R. S. & Cosner C. (1999) How habitat edges change species interactions. American Naturalist 153: 165–182.
Gauch Jr H. G. (1982) Multivariate Analysis in Community Ecology. Cambridge University Press, Cambridge.
Grant B. R. & Grant P. R. (1981) Exploitation of Opuntia cactus by birds on the Galápagos. Oecologia 49: 179–187.
Gray L. J. (1993) Response of insectivorous birds to emerging aquatic insects in riparian habitats of a tallgrass prairie stream. American Midland Naturalist 129: 288–300.
Greensberg R. (1987) Seasonal foraging specialization in the worm-eating warbler. Condor 89: 158–168.
Gunnarsson B. (1996) Bird predation and vegetation structure affecting spruce-living arthropods in a temperate forest. Journal of Animal Ecology 65: 389–397.
Holmes R. T., Shultzs J. C. & Nothnagle P. (1979) Bird predation on forest insects: An exclosure experiment. Science 206: 462–463.
Huxel G. R. & McCann K. (1998) Food web stability: The influence of trophic flows across habitats. American Naturalist 152: 460–469.
Kendeigh S. C. (1944) Measurements of bird populations. Ecological Monographs 14: 67– 106.
Lack D. (1971).Ecological Isolation in Birds. Harvard University Press, Cambridge, MA.
Larue P., Bélanger L. & Huot J. (1995) Riparian edge effects on boreal balsam fir bird communities. Canadian Journal of Forest Research 25: 555–566.
Miles D. B. (1990a) The importance and consequences of temporal variation in avian foraging behavior. Studies Avian Biology 13: 210–217.
Miles D. B. (1990b) A comparison of three multivariate statistical techniques for the analysis of avian foraging data. Studies Avian Biology 13: 295–308.
Moreno E. & Carrascal L. M. (1993) Leg morphology and feeding postures in four Parus species: An experimental ecomorphological approach. Ecology 74: 2037–2044.
Murakami M. & Nakano S. (2000) Bird function in a forest canopy food web. Proceedings of the Royal Society of London, Biological Science 267: 1597–1601.
Naiman R. J., Décamps H. & Pollock M. (1993) The role of riparian corridors in maintaining regional biodiversity. Ecological Application 3: 209–212.
Nakano S., Miyasaka H. & Kuhara N. (1999) Terrestrial–aquatic linkages: Riparian arthropod inputs alter trophic cascades in a stream food web. Ecology 80: 2435–2441.
Nakano S. & Murakami M. (2001) Reciprocal subsidies: Dynamic interdependence between terrestrial and aquatic food webs. Proceedings of the National Academy of Science of the United States of America 91: 166–170.
Polis G. A., Anderson W. B. & Holt R. D. (1997) Toward an integration of landscape and food web ecology: The dynamics of spatially subsidized food webs. Annual Review of Ecology and Systematics 28: 289–316.
Polis G. A. & Hurd S. D. (1996a) Allochthonous input across habitats, subsidized consumers, and apparent trophic cascades: Examples from the ocean–land interface. In: Food Webs: Integration of Patterns and Dynamics. (eds G. A. Polis & K. O. Winemiller) pp. 275–285. Chapman & Hall, New York.
Polis G. A. & Hurd S. D. (1996b) Linking marine and terrestrial food webs: Allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. American Naturalist 147: 396– 423.
Power M. E. & Rainey W. E. (2000) Food webs and resource sheds: Towards spatially delimiting trophic interactions. In: The Ecological Consequences of Environmental Heterogeneity. (eds M. J. Hutchings, E. A. John & A. J. A. Stewart) pp. 291–314. Blackwell Science Inc, Oxford.
Schluter D. (1982) Seed and patch selection by Galápagos ground finches; relation to foraging efficiency and food supply. Ecology 63: 1106–1120.
Smith J. N. M., Grant P. R., Grant B. R., Abbot I. J. & Abbot L. K. (1978) Seasonal variation in feeding habitats of Darwin’s ground finches. Ecology 59: 1137–1150.
Smith K. G. & Rotenberry J. T. (1990) Quantifying food resources in avian studies: Present problems and future needs. Studies Avian Biology 13: 3–5.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Murakami, M., Nakano, S. Species-specific foraging behavior of birds in a riparian forest. Ecol Res 16, 913–923 (2001). https://doi.org/10.1046/j.1440-1703.2001.00448.x
Accepted:
Issue Date:
DOI: https://doi.org/10.1046/j.1440-1703.2001.00448.x