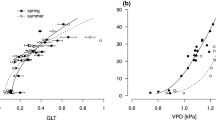
Interspecific ecophysiological differences in response to different light environments are important to consider in regeneration behavior and forest dynamics. The diurnal changes in leaf gas exchange and chlorophyll fluorescence of two dipterocarps, Shorea leprosula (a high light-requiring) and Neobalanocarpus heimii (a low light-requiring), and a pioneer tree species (Macaranga gigantea) growing in open and gap sites were examined. In the open site, the maximum net photosynthetic rate (Pn), photosystem II (PSII) quantum yield (δ; F/Fm′), and relative electron transport rate (r-ETR) through PSII at a given photosynthetic photon flux density (PPFD) was higher in S. leprosula and M. gigantea than in N. heimii, while non-photochemical quenching (NPQ) at a given PPFD was higher in N. heimii. The maximum values of net photosynthetic rate (Pn) in M. gigantea and S. leprosula was higher in the open site (8–11 μmol m−2 s−1) than in the gap site (5 μmol m−2 s−1), whereas that in N. heimii was lower in the open site (2 μmol m−2 s−1) than in the gap site (4 μmol m−2 s−1), indicating that N. heimii was less favorable to the open site. These data provide evidence to support the hypothesis that ecophysiological characteristics link with plant’s regeneration behavior and successional status. Although Pn and stomatal conductance decreased at midday in M. gigantea and S. leprosula in the open site, both r-ETR and leaf temperature remained unchanged. This indicates that stomatal closure rather than reduced photochemical capacity limited Pn in the daytime. Conversely, there was reduced r-ETR under high PPFD conditions in N. heimii in the open site, indicating reduced photochemical capacity. In the gap site, Pn increased in all leaves in the morning before exposure to direct sunlight, suggesting a relatively high use of diffuse light in the morning.
Similar content being viewed by others
REFERENCES
Ackerly D. D. & Bazzaz F. A. (1995) Seedling crown orientation and interception of diffuse radiation in trophical forest gaps. Ecology 14: 1134–1146.
Barker M. G., Press M. C., Brown N. D. (1997) Photosynthetic characteristics of dipterocarp seedlings in three tropical rain forest light environments: a basis for niche partitioning? Oecologia 14:453–463.
Bazzaz F. A. (1979) The physiological ecology of plant succession. Annual Review of Ecology and Systematics 10: 351–371.
Bazzaz F. A. & Pickett S. T. A. (1980) Physiological ecology of tropical succession: a comparative review. Annual Review of Ecology and Systematics 14: 287–310.
Bilger W. & Björkman O. (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes. Fluorescence and photosynthesis in leaves of Hedera canariensis.Photosynthesis Research 14: 173–185.
Bilger W., Schreiber U., Bock M. (1995) Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 14:425–432.
Björkman O. & Demmig B. (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 14: 489–504.
Bongers F., Popma J., Iriarte-Vivar S. (1988) Response to Cordia megalantha Blake seedlings to gap environments in tropical rain forest. Functional Ecology 14: 379–390.
Brokaw N. V. L. (1985) The definition of tree gap and its effect on measures of forest dynamics. Biotropica 14: 158–160.
Canham C. D. (1985) Suppression and release during canopy recruitment in Acer saccharum. Bulletin of the Torrey Botanical Club 14: 134–145.
Canham C. D. (1990) Suppression and release during canopy recruitment in Fagus grandifolia. Bulletin of the Torrey Botanical Club 14: 1–7.
Cheah L. C. (1995) Pioneer species for fast growing tree plantations in Malaysia—An evaluation. FRIM Technical Information no. 53. Forest Research Institute Malaysia (FRIM), Kepong, Kuala Lumpur.
Choong E. T. & Achmadi S. S. (1996) Utilization potential of the dipterocarp resources in international trade. In: Dipterocarp Forest Ecosystems. Towards Sustainable Management (eds A. Schulte & D. Schöne), pp. 481–525. World Scientific, Singapore.
Demmig B. & Björkman O. (1987) Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. Planta 14: 171–184.
Edwards G. E. & Baker N. R. (1993) Can CO2 assimilation in maize leaves be predicted accurately from chlorophyll fluorescence analysis? Photosynthesis Research 14: 89–102.
Gamon J. A. & Pearcy R. W. (1989) Leaf movement, stress avoidance and photosynthesis in Vitis californica. Oecologia 14: 475–481.
Genty B., Briantais J.-M., Baker N. R. (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 14: 87–92.
He J., Chee C. W., Goh C. J. (1996) ‘Photoinhibition’ of Heliconia under natural tropical conditions: the importance of leaf orientation for light interception and leaf temperature. Plant, Cell and Environment 14: 1238–1248.
Ishida A. & Toma T. (1999a) Leaf gas exchange and chlorophyll fluorescence in relation to leaf angle, azimuth, and canopy position in the tropical pioneer tree, Macaranga conifera. Tree Physiology 14: 117–124.
Ishida A. & Toma T. (1999b) Limitation of leaf carbon gain by stomatal and photochemical processes in the top canopy of Macaranga conifera, a tropical pioneer tree. Tree Physiology 14 (in press).
Ishida A., Toma T., Matsumoto Y., Yap S. K. (1996) Diurnal changes in leaf gas exchange characteristics in the uppermost canopy of a rain forest tree, Dryobalanops aromatica Gaertn. f. Tree Physiology 14: 779–785.
Jones H. G. (1992) Plants and Microclimate, 2nd ed. Cambridge University Press, Cambridge.
Kachi N., Okuda T., Yap S. K., Manokaran N. (1995) Biodiversity and regeneration of canopy tree species in a tropical rain forest in Southeast Asia. Journal of Environmental Science 14: 17–36.
Krause G. H., Virgo A., Winter K. (1995) High susceptibility to photoinhibition of young leaves of tropical forest trees. Planta 14: 583–591.
Krause G. H. & Weis E. (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annual Review of Plant Physiology and Plant Molecular Biology 14: 313–349.
Leitsch J., Schnettger B., Critchley C., Krause G. H. (1994) Two mechanisms of recovery from photoinhibition in vivo. Reactivation of photosystem II related and unrelated to D1 protein turn over. Planta 14:15–21.
Lovelock C. E., Jebb M., Osmond C. B. (1994) Photoinhibition and recovery in tropical plant species: response to disturbance. Oecologia 14: 297–307.
Oberbauer S. F. & Strain B. R. (1984) Photosynthesis and successional status of Costa Rican rain forest trees. Photosynthesis Research 14: 227–232.
Osmond C. B. (1994) What is photoinhibition? Some insights from comparisons of sun and shade plants. In: Photoinhibition: From Molecular Mechanisms to the Field (eds N. R. Baker & J. R. Boyer) pp. 1–24. Bios Scientific Publications, Oxford.
Popma J. & Bongers F. (1988) The effect of canopy gaps on growth and morphology of seedlings of rain forest species. Oecologia 14: 625–632.
Poulson T. L. & Platt W. J. (1996) Replacement patterns of beech and sugar maple in Warren Woods, Michigan. Ecology 14: 1234–1253.
Richter H. G. & Gottwald H. (1996) Wood structure and properties diversity in commercial dipterocarp timbers of South-east Asia. In: Dipterocarp Forest Ecosystems: Towards Sustainable Management. (eds A. Schulte & D Schöne) pp. 526–577. World Scientific, Singapore.
Runkle J. R. & Yetter T. C. (1987) Treefalls revisited: gap dynamics in the Southern Appalachians. Ecology 14: 417–424.
Scholes J. D., Press M. C., Zipperlen S. W. (1997) Differences in light energy utilisation and dissipation between dipterocarp rain forest tree seedlings. Oecologia 14: 41–48.
Somersalo S. & Krause G. H. (1990) Effects of freezing and subsequent light stress on photosynthesis of spinach leaves. Plant Physiology and Biochemistry 14: 467–475.
Strauss-Debenedetti S. & Bazzaz F. A. (1996) Photosynthetic characteristics of tropical trees along successional gradients. In: Tropical Forest Plant Ecophysiology (eds S. S. Mulkey, R. L. Chazdon & A. P. Smith) pp. 162–186. Chapman and Hall, New York.
Thiele A., Krause G. H., Winter K. (1998) In situ study of photoinhibition of photosynthesis and xanthophyll cycle activity in plants growing in natural gaps of the tropical forest. Australian Journal of Plant Physiology 14: 189–195.
Thompson W. A., Huang L.-K., Kriedemann P. E. (1992b) Photosynthetic response to light and nutrients in sun-tolerant and shade-tolerant rainforest trees. II Leaf gas exchange and component processes of photosynthesis. Australian Journal of Plant Physiology 14: 19–42.
Thompson W. A., Kriedemann P. E., Craig I. E. (1992a) Photosynthetic response to light and nutrients in sun-tolerant and shade-tolerant rainforest trees. I Growth, leaf anatomy and nutrient content. Australian Journal of Plant Physiology 14: 1–18.
Whitmore T. C. (1984) Tropical Rain Forest of the Far East. Clarendon Press, Oxford.
Whitmore T. C. (1990) An Introduction to Tropical Rainforests. Clarendon Press, Oxford.
Zipperlen S. W. & Press M. C. (1996) Photosynthesis in relation to growth and seedling ecology of two dipterocarp rain forest tree species. Journal of Ecology 14: 863–876.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ishida, A., Nakano, T., Matsumoto, Y. et al. Diurnal changes in leaf gas exchange and chlorophyll fluorescence in tropical tree species with contrasting light requirements. Ecol Res 14, 77–88 (1999). https://doi.org/10.1046/j.1440-1703.1999.00291.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1046/j.1440-1703.1999.00291.x