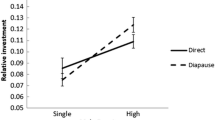
In mating of the dobsonfly, Protohermes grandis (Thunberg), the male attaches the spermatophore externally to the female genitalia. The spermatophore includes a large gelatinous mass which the female detaches and feeds on after mating. While the female consumes this nuptial food gift, sperm is evacuated from the remaining portion of the spermatophore (sperm package) into her reproductive tract. Under laboratory conditions, mated females maintained receptivity throughout their lifetime, and they remated even on the day following copulation. A single insemination may supply enough sperm, as females mated only once deposited fertile eggs throughout life and, when dissected after death, all females had sperm in the spermatheca. There was a positive correlation between longevity and the number of matings. Lifetime fecundity also increased as mating multiplied. However, the size of eggs and hatchlings was not influenced by the number of matings. It seems that large spermatophore consumption by female P. grandis provides nutrients that increase fitness not in offspring quality, but in their quantity.
Similar content being viewed by others
References
Boggs C. L. (1990) A general model of the role of male-donated nutrients in female insects’ reproduction. American Naturalist 136: 598–617.
Drummond B. A. (1984) Multiple mating and sperm competition in the Lepidoptera. In: Sperm Competition and the Evolution of Animal Mating Systems (ed R. L. Smith) pp. 291–370. Academic Press, New York.
Eberhard W. G. (1996) Female Control: Sexual Selection by Cryptic Female Choice. Princeton University Press, Princeton.
Fox C. W. (1993) Multiple mating, lifetime fecundity and female mortality of the bruchid beetle, Callosobruchus maculatus (Coleoptera: Bruchidae). Functional Ecology 7: 203–208.
Gwynne D. T. (1983) Male nutritional investment and the evolution of sexual differences in Tettigoniidae and other Orthoptera. In: Orthopteran Mating Systems (eds D. T. Gwynne & G. K. Morris) pp. 337–366. Westview Press, Colorado.
Gwynne D. T. (1984) Courtship feeding increases female reproductive success in bushcrickets. Nature 307: 361–363.
Gwynne D. T. (1988) Courtship feeding and the fitness of female katydids (Orthoptera: Tettigoniidae). Evolution 42: 545–555.
Halliday T. & Arnold S. J. (1987) Multiple mating by females: a perspective from quantitative genetics. Animal Behaviour 35: 939–941.
Hayashi F. (1988) Prey selection by the dobsonfly larva, Protohermes grandis (Megaloptera: Corydalidae). Freshwater Biology 20: 19–29.
Hayashi F. (1992) Large spermatophore production and consumption in dobsonflies Protohermes (Megaloptera, Corydalidae). Japanese Journal of Entomology 60: 59–66.
Hayashi F. (1993) Male mating costs in two insect species (Protohermes, Megaloptera) that produce large spermatophores. Animal Behaviour 45: 343–349.
Hayashi F. (1994) Life-history patterns in 15 populations of Protohermes (Megaloptera: Corydalidae): effects of prey size and temperature. In: Insect Life-cycle Polymorphism (ed. H. V. Danks) pp. 227–243. Kluwer Academic Publishers, Dordrecht.
Heller K-G. & Helversen D. (1991) Operational sex ratio and individual mating frequencies in two bushcricket species (Orthoptera, Tettigonioidea, Poecilimon). Ethology 89: 211–228.
Heller K-G. & Reinhold K. (1994) Mating effort function of the spermatophore in the bushcricket Poecilimon veluchianus (Orthoptera, Phaneropteridae): support from a comparison of the mating behaviour of two subspecies. Biological Journal of the Linnean Society 53: 153–163.
Jennions M. D. (1997) Female promiscuity and genetic incompatibility. Trends in Ecology and Evolution 12: 251–253.
Jones K. N., Odendaal F. J., Ehrlich P. R. (1986) Evidence against the spermatophore as paternal investment in checkerspot butterflies (Euphydryas: Nymphalidae). American Midland Naturalist 116: 1–6.
KASUYA E. & SATO N. (1998) Effects of the consumption of male spermatophylax on the oviposition schedule of females in the decorated cricket, Gryllodes sigillatus. Zoological Science15: 127–130.
Matsuzaki M., Sasaki A., Komuro M. (1985) Panoistic ovarioles of the dobsonfly, Protohermes grandis (Megaloptera, Corydalidae). In: Recent Advances in Insect Embryology in Japan (eds H. Ando & K. Miya) pp. 13–23. ISEBU Co. Ltd, Tsukuba.
Parker G. A. & Simmons L. W. (1989) Nuptial feeding in insects: theoretical models of male and female interests. Ethology 82: 3–26.
Reinhold K. & Heller K-G. (1993) The ultimate function of nuptial feeding in the bushcricket Poecilimon veluchianus (Orthoptera: Tettigoniidae: Phaneropterinae). Behavioral Ecology and Sociobiology 32: 55–60.
Ridley M. (1988) Mating frequency and fecundity in insects. Biological Review 63: 509–549.
Sakaluk S. K. (1984) Male crickets feed females to ensure complete sperm transfer. Science 223: 609–610.
Sakaluk S. K. (1985) Spermatophore size and its role in the reproductive behaviour of the cricket, Glyllodes supplicans (Orthoptera: Gryllidae). Canadian Journal of Zoology 63: 1652–1656.
Sakaluk S. K. (1997) Cryptic female choice predicted on wing dimorphism in decorated crickets. Behavioral Ecology 8: 326–331.
Simmons L. W. (1990) Nuptial feeding in tettigoniids: male costs and the rates of fecundity increase. Behavioral Ecology and Sociobiology 27: 43–47.
Simmons L. W., Teale R. J., Maier M., Standish R. J., Bailey W. J., Withers P. C. (1992) Some costs of reproduction for male bushcrickets, Requena verticalis (Orthoptera: Tettigoniidae): allocating resources to mate attraction and nuptial feeding. Behavioral Ecology and Sociobiology 31: 57–62.
Sväd L. & Wiklund C. (1991) The effect of ejaculate mass on female reproductive output in the European swallowtail butterfly, Papilio machaon (L.) (Lepidoptera: Papilionidae). Journal of Insect Behavior 4: 33–41.
Thornhill R. & Alcock J. (1983) The Evolution of Insect Mating Systems. Harvard University Press, Cambridge.
Vahed K. (1998) The function of nuptial feeding in insects: a review of empirical studies. Biological Review 73: 43–78.
Vahed K. & Gilbert F. S. (1997) No effect of nuptial gift consumption on female reproductive output in the bushcricket Leptophyes laticauda Friv. Ecological Entomology 22: 479–482.
Wedell N. & Arak A. (1989) The wartbiter spermatophore and its effect on female reproductive output (Orthoptera: Tettigoniidae, Decticus verrucivorus). Behavioral Ecology and Sociobiology 24: 117–125.
Will M. W. & Sakaluk S. K. (1994) Courtship feeding in decorated crickets: is the spermatophylax a sham? Animal Behaviour 48: 1309–1315.
Yasui Y. (1998) The ‘genetic benefits’ of female multiple mating reconsidered. Trends in Ecology and Evolution 13: 246–250.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hayashi, F. Multiple mating and lifetime reproductive output in female dobsonflies that receive nuptial gifts. Ecol Res 13, 283–289 (1998). https://doi.org/10.1046/j.1440-1703.1998.00272.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1046/j.1440-1703.1998.00272.x