Abstract
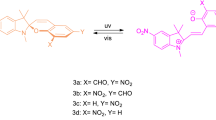
Five new N-phenyl-carbazole benzopyrans bearing different substitutions on one of the phenyl rings at the sp3 carbon have been synthesized. Their molecular structures were investigated by X-ray and NMR analyses and through quantum chemical calculations. The photochromic mechanism under UV irradiation in toluene, consisting of the consecutive formation of transoid-cis (TC) and transoid-trans (TT) isomers, was studied by UV-vis spectral and kinetic analyses. These molecules have been specifically designed to ascertain the possibility of favouring the formation of the less thermodynamically stable TT at the photostationary state, upon exploiting steric hindrance effects on the diene part of the molecule. The spectrokinetic study allowed the estimation of most of the spectrokinetic parameters, such as molar extinction coefficients, quantum yields of UV colouration and visible photobleaching, and the rate constants of the fast and slow thermal bleaching processes. Peculiar effects of substituents with different donor strengths on one phenyl ring located at the 3-position were observed on the spectrokinetic properties.
Similar content being viewed by others
References
B. Van Gemert, in Organic Photochromic and Thermochromic Compounds, ed. J. C. Crano and R. J. Guglielmetti, Kluwer Academic/Plenum Publishers, New York, 1999, vol. 1, pp. 111–140.
J. D. Hepworth and B. M. Heron, in Functional Dyes, ed. S.-H. Kim, Amsterdam, Elsevier, 2006, pp. 85–135.
N. S. Corns, S. M. Partington and A. D. Towns, Industrial organic photochromic dyes, Color. Technol., 2009, 125, 249–261.
J. Kolc and R. S. Becker, Proof of structure of the colored photoproducts of chromenes and spiropyrans, J. Phys. Chem., 1967, 71, 4045–4048.
C. Lenoble and R. S. Becker, Photophysics, photochemistry and kinetics of photochromic 2H-pyrans and chromenes, J. Photochem., 1986, 33, 187–197.
H. Bouas-Laurent and H. Dürr, Organic photochromism, Pure Appl. Chem., 2001, 73, 639–665.
G. Ottavi, G. Favaro and V. Malatesta, Spectrokinetic study of 2,2–diphenyl-5,6-benzo(2H)chromene: a thermoreversible and photoreversible photochromic system, J. Photochem. Photobiol., A, 1998, 115, 123–128.
S. Delbaere, G. Vermeersch and J.-C. Micheau, Quantitative analysis of the dynamic behaviour of photochromic systems, J. Photochem. Photobiol., C, 2011, 12, 74–105.
R Demadrille, A. Rabourdin, M. Campredon and G. Giusti, Spectroscopic characterisation and photodegradation studies of photochromic spiro[fluorene-9,3′-[3′H]-naphtho[2,1-b] pyrans], J. Photochem. Photobiol., A, 2004, 168, 143–152.
C. D. Gabbutt, M. Heron, A. C. Instone, S. B. Kolla, K. Mahajan, P. J. Coelho and L. M. Carvalho, Synthesis and photochromic properties of symmetrical aryl ether linked bi- and tri-naphthopyrans, Dyes Pigm., 2008, 76, 24–34.
R. S. Becker and J. Michl, Photochromism of Synthetic and Naturally Occurring 2H-Chromenes and 2H-Pyrans, J. Am. Chem. Soc., 1966, 88, 5931–5933.
B. Luccioni-Houze, M. Campredon, R. Guglielmetti and G. Giusti, Kinetic Analysis of Fluoro-[2 h]-Chromenes at the Photostationary States, Mol. Cryst. Liq. Cryst., 1997, 297, 161–165.
J. J. Luthem, The Effect of Substituents on the Ring Closure Reaction of Photo Activated Diarylnaphthopyrans, Mol. Cryst. Liq. Cryst., 1997, 297, 155–160.
A. Kumar, The Relationship Between the Structure and the Absorption Spectra of Naphtho[2,1-B]pyran, Mol. Cryst. Liq. Cryst., 1997, 297, 139–145.
J. Hobley, V. Malatesta, R. Millini, W. Giroldini, L. Wis, M. Goto, M. Kishimotoa and H. Fukumura, Photochromism of chromene crystals; a new property of old chromenes, Chem. Commun., 2000, 1339–1340.
J. Aubard, F. Maurel, G. Buntinx, O. Poizat, G. Levi, R. Guglielmetti and A. Samat, Femto/Picosecond Transient Absorption Spectroscopy of Photochromic 3,3-Diphenylnaphtho[2,1-b]pyran, Mol. Cryst. Liq. Cryst., 2000, 345, 215–220.
S. Delbaere and G. Vermeersch, NMR characterization of allenyl-naphthol in the photochromic process of 3,3-diphenyl-[3H]-naphtho[2-1,b]pyran, J. Photochem. Photobiol., A, 2003, 159, 227–232.
P. L. Gentili, E. Danilov, F. Ortica, M. A. J. Rodgers and G. Favaro, Dynamics of the excited states of chromenes studied by fast and ultrafast spectroscopies, Photochem. Photobiol. Sci., 2004, 3, 886–891.
R. G. Brown, M. Maafl, P. Foggi and L. Bussotti, The ultrafast dynamics of some photochromic naphthopyrans, in Femtochemistry and Femtobiology, ed. M. M. Martin and J. T. Hynes, 2004, 283–286.
F. Maurel, S. Lau Truong, J. P. Bertigny, R. Dubest, G. Lévi, J. Aubard, S. Delbaere and G. Vermeersch, SERS Study of 3,3-Diphenyl-naphtho[2,1-b]pyran: Another Evidence for Allenyl-Naphthol Involvement in the Photochromic Mechanism, Mol. Cryst. Liq. Cryst., 2005, 430, 235–241.
B. Moine, G. Buntinx, O. Poizat, J. Rehault, C. Moustrou and A. Samat, Transient absorption investigation of the photophysical properties of new photochromic 3H-naphtho [2,1-b]pyran, J. Phys. Org. Chem., 2007, 20, 936–943.
B. Moine, J. Réhault, S. Aloïse, J.-C. Micheau, C. Moustrou, A. Samat, O. Poizat and G. Buntinx, Transient Absorption Studies of the Photochromic Behavior of 3H-Naphtho[2,1-b]pyrans Linked to Thiophene Oligomers via an Acetylenic Junction, J. Phys. Chem. A, 2008, 112, 4719–4726.
J. Harada, K. Ueki, M. Anada, Y. Kawazoe and K. Ogawa, Solid-state Photochromism of Chromenes: Enhanced Photocoloration and Observation of Unstable Colored Species at Low Temperatures, Chem. – Eur. J., 2011, 17, 14111–14119.
T. T. Herzog, G. Ryseck, E. Ploetz and T. Cordes, The photochemical ring opening reaction of chromene as seen by transient absorption and fluorescence spectroscopy, Photochem. Photobiol. Sci., 2013, 12, 1202–1209.
Y. Inagaki, Y. Kobayashi, K. Mutoh and J. Abe, A Simple and Versatile Strategy for Rapid Color Fading and Intense Coloration of Photochromic Naphthopyran Families, J. Am. Chem. Soc., 2017, 139, 13429–13441.
S. Brazevic, S. Nizinski, R. Szabla, M. F. Rode and G. Burdzinski, Photochromic reaction in 3H-naphthopyrans studied by vibrational spectroscopy and quantum chemical calculations, Phys. Chem. Chem. Phys., 2019, 21, 11861–11870.
S. Brazevic, M. Baranowski, M. Sikorski, M. Rode and G. Burdzinski, Ultrafast dynamics of the transoid-cis isomer formed in photochromic reaction from 3H-naphthopyran, ChemPhysChem, 2020, 21, 1402–1407.
C. M. Sousa, J. Berthet, S. Delbaere and P. J. Coelho, Synthesis of 1-Vinylidene-naphthofurans: A Thermally Reversible Photochromic System That Colors Only When Adsorbed on Silica Gel, J. Org. Chem., 2013, 78, 6956–6961.
C. Sousa, S. Saraiva, H. Macedo and P. Coelho, Grey colouring thermally reversible photochromic 1-vinylidenenaphthofurans, Dyes Pigm., 2017, 141, 269–276.
C. M. Sousa, J. Berthet, S. Delbaere, A. Polonia and P. J. Coelho, Control of the Switching Speed of Photochromic Naphthopyrans through Restriction of Double Bond Isomerization, J. Org. Chem., 2017, 82, 12028–12037.
C. M. Sousa and P. J. Coelho, Joining High Coloration and Fast Color Fading with Photochromic Fused-Naphthopyrans, Eur. J. Org. Chem., 2020, 985–992.
H. Kuroiwa, Y. Inagaki, K. Mutoh and J. Abe, On-Demand Control of the Photochromic Properties of Naphthopyrans, Adv. Mater., 2019, 31, 1805661.
M. Frigoli, F. Maurel, J. Berthet, S. Delbaere, J. Marrot and M. M. Oliveira, The Control of Photochromism of [3H]-Naphthopyran Derivatives with Intramolecular CH-π Bonds, Org. Lett., 2012, 14, 4150–4153.
M. Frigoli, J. Marrot, P. L. Gentili, D. Jacquemin, M. Vagnini, D. Pannacci and F. Ortica, P-type Photochromism of new Helical Naphthopyrans: Synthesis, and Photochemical, Photophysical and Theoretical Study, ChemPhysChem, 2015, 16, 2447–2458.
F. Ianni, S. Scorzoni, P. L. Gentili, A. Di Michele, M. Frigoli, E. Camaioni, F. Ortica and R. Sardella, Chiral separation of helical chromenes with chloromethyl phenylcarbamate polysaccharide-based stationary phases, J. Sep. Sci., 2018, 41, 1266–1273.
S. R. Meech and D. Phillips, Photophysics of some common fluorescence standards, J. Photochem., 1983, 23, 193–217.
M. M. Oliveira, M. A. Salvador, P. J. Coelho and L. M. Carvalho, New benzopyranocarbazoles: synthesis and photochromic behavior, Tetrahedron, 2005, 61, 1681–1691.
J. C. Antilla, A. Klapars and S. L. Buchwald, The Copper-Catalyzed N-Arylation of Indoles, J. Am. Chem. Soc., 2002, 124, 11684–11688.
J. Pang, Y. Tao, S. Freiberg, X.-P. Yang, M. D’Iorioa and S. Wang, Syntheses, structures, and electroluminescence of new blue luminescent star-shaped compounds based on 1,3,5-triazine and 1,3,5-trisubstituted benzene, J. Mater. Chem., 2002, 12, 206–212.
M. M. Oliveira, M. A. Salvador, S. Delbaere, J. Berthet, G. Vermeersch, J. C. Micheau, P. J. Coelho and L. M. Carvalho, Remarkable thermally stable open forms of photochromic new N-substituted benzopyranocarbazoles, J. Photochem. Photobiol., A, 2008, 198, 242–249.
P. R. Andrews, S. L. A. Munro, M. Sadek and M. G. Wong, The hybridization state of nitrogen as a conformational variable in biologically active molecules, J. Chem. Soc., Perkin Trans. 2, 1988, 711–718.
I. B. Berlman, Handbook of fluorescence spectra of aromatic molecules, Academic Press, 1971, p. 213.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available. CCDC 1998515. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0pp00202j
Rights and permissions
About this article
Cite this article
Frigoli, M., Jousselin-Oba, T., Mamada, M. et al. Synthesis and photochromic behaviour of a series of benzopyrans bearing an N-phenyl-carbazole moiety: photochromism control by the steric effect. Photochem Photobiol Sci 19, 1344–1355 (2020). https://doi.org/10.1039/d0pp00202j
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/d0pp00202j