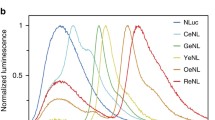
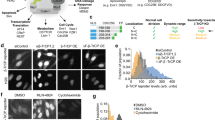
Abstract
Cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB) is associated with memory formation and controls cell survival and proliferation via regulation of downstream gene expression in tumorigenesis. As a transcription factor, CREB binds to cAMP response elements. Phosphorylation of CREB triggers transcriptional activation of CREB downstream genes following the interaction of the kinase-inducible domain (KID) of CREB with the KID interaction domain (KIX) of CREB-binding protein. Nevertheless, because of the lack of single-cell analytical techniques, little is known about spatiotemporal regulation of CREB phosphorylation. To analyze CREB activation in single living cells, we developed genetically encoded bioluminescent sensors using luciferase-fragment complementation: the sensors are designed based on KID–KIX interaction with a single-molecule format. The luminescence intensity of the sensor, designated as CREX (a sensor of CREB activation based on KID (CREB)–KIX interaction), increased by phosphorylation of CREB. Moreover, the luminescence intensity of CREX was sufficient to detect CREB activation in live-cell bioluminescence imaging for single-cell analysis because of the higher sensitivity. CREX sensor is expected to contribute to elucidation of the spatiotemporal regulation of CREB phosphorylation by applying single-cell analysis.
Similar content being viewed by others
References
M. R. Montminy and L. M. Bilezikjian, Nature, 1987, 328, 175–178.
E. Benito and A. Barco, Trends Neurosci., 2010, 33, 230–240.
F. Lu, Y. Zheng, P. O. Donkor, P. Zou and P. Mu, Oncol. Res., 2016, 24, 171–179.
A. Steven and B. Seliger, Oncotarget, 2016, 7, 35454–35465.
A. Steven, M. Heiduk, C. V. Recktenwald, B. Hiebl, C. Wickenhauser, C. Massa and B. Seliger, Mol. Cancer Res., 2015, 13, 1248–1262.
G. A. Gonzalez and M. R. Montminy, Cell, 1989, 59, 675–680.
P. Sun, H. Enslen, P. S. Myung and R. A. Maurer, Genes Dev., 1994, 8, 2527–2539.
J. Xing, D. D. Ginty and M. E. Greenberg, Science, 1996, 273, 959–963.
M. Johannessen, M. P. Delghandi and U. Moens, Cell. Signalling, 2004, 16, 1211–1227.
J. C. Chrivia, R. P. S. Kwok, N. Lamb, M. Hagiwara, M. R. Montminy and R. H. Goodman, Nature, 1993, 365, 855–859.
D. Parker, K. Ferreri, T. Nakajima, V. J. LaMorte, R. Evans, S. C. Koerber, C. Hoeger and M. R. Montminy, Mol. Cell. Biol., 1996, 16, 694–703.
B. M. Mayr, G. Canettieri and M. R. Montminy, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 10936–10941.
M. W. Friedrich, G. Aramuni, M. Mank, J. A. G. Mackinnon and O. Griesbeck, J. Biol. Chem., 2010, 285, 23285–23295.
T. Ozawa, H. Yoshimura and S. B. Kim, Anal. Chem., 2013, 85, 590–609.
T. Ishimoto, H. Mano, T. Ozawa and H. Mori, Bioconjugate Chem., 2012, 23, 923–932.
H. Mano, T. Ishimoto, T. Okada, N. Toyooka and H. Mori, Biol. Pharm. Bull., 2014, 37, 1689–1693.
T. Ishimoto, K. Azechi and H. Mori, Biol. Pharm. Bull., 2015, 38, 1969–1974.
G. Merutka, W. Shalongo and E. Stellwagen, Biochemistry, 1991, 30, 4245–4248.
M. Sato, Y. Ueda, T. Takagi and Y. Umezawa, Nat. Cell Biol., 2003, 5, 1016–1022.
K. J. Herbst, M. D. Allen and J. Zhang, PLoS One, 2009, 4, e5642.
M. D. Conkright, G. Canettieri, R. Screaton, E. Guzman, L. Miraglia, J. B. Hogenesch and M. Montminy, Mol. Cell, 2003, 12, 413–423.
R. A. Screaton, M. D. Conkright, Y. Katoh, J. L. Best, G. Canettieri, S. Jeffries, E. Guzman, S. Niessen, J. R. Yates 3rd, H. Takemori, M. Okamoto and M. Montminy, Cell, 2004, 119, 61–74.
C. Hollands, N. Bartolotti and O. Lazarov, Front. Neurosci., 2016, 10, 178.
T. Ishimoto, H. Mano and H. Mori, Sci. Rep., 2015, 5, 9757.
K. H. Lee, S. S. Byun, J. Y. Paik, S. Y. Lee, S. H. Song, Y. S. Choe and B. T. Kim, Nucl. Med. Commun., 2003, 24, 1003–1009.
D. M. Mofford and S. C. Miller, ACS Chem. Neurosci., 2015, 6, 1273–1275.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available. See DOI: 10.1039/c9pp00249a
Rights and permissions
About this article
Cite this article
Noda, N., Ishimoto, T., Mori, H. et al. Enhanced bioluminescent sensor for longitudinal detection of CREB activation in living cells. Photochem Photobiol Sci 18, 2740–2747 (2019). https://doi.org/10.1039/c9pp00249a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c9pp00249a