Abstract
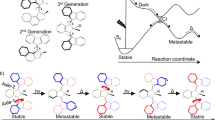
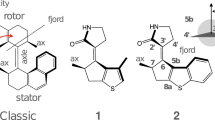
A molecular motor potentially performing a continuous unidirectional rotation is studied by a multidisciplinary approach including organic synthesis, transient spectroscopy and excited state trajectory calculations. A stereogenic center was introduced in the N-alkylated indanylidene–pyrroline Schiff base framework of a previously investigated light-driven molecular switch in order to achieve the unidirectional C=C rotary motion typical of Feringa’s motor. Here we report that the specific substitution pattern of the designed chiral molecule must critically determine the unidirectional efficiency of the light-induced rotary motion. More specifically, we find that a stereogenic center containing a methyl group and a hydrogen atom as substituents does not create a differential steric effect large enough to fully direct the motion in either the clockwise or counterclockwise direction especially along the E → Z coordinate. However, due to the documented ultrafast character and electronic circular dichroism activity of the investigated system, we find that it provides the basis for development of a novel generation of rotary motors with a biomimetic framework and operating on a picosecond time scale.
Similar content being viewed by others
Notes and references
D. Roke, S. J. Wezenberg and B. L. Feringa, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 9423–9431.
R. D. Astumian, Chem. Sci., 2017, 8, 840–845.
S. Erbas-Cakmak, S. D. P. Fielden, U. Karaca, D. A. Leigh, C. T. McTernan, D. J. Tetlow and M. R. Wilson, Science, 2017, 358, 340–343.
B. S. L. Collins, J. C. M. Kistemaker, E. Otten and B. L. Feringa, Nat. Chem., 2016, 8, 860.
C. R. Hall, J. Conyard, I. A. Heisler, G. Jones, J. Frost, W. R. Browne, B. L. Feringa and S. R. Meech, J. Am. Chem. Soc., 2017, 139, 7408–7414.
J. C. M. Kistemaker, P. Štacko, J. Visser and B. L. Feringa, Nat. Chem., 2015, 7, 890.
N. Koumura, R. W. J. Zijlstra, R. A. van Delden, N. Harada and B. L. Feringa, Nature, 1999, 401, 152–155.
C. Schnedermann, X. Yang, M. Liebel, K. M. Spillane, J. Lugtenburg, I. Fernández, A. Valentini, I. Schapiro, M. Olivucci, P. Kukura and R. A. Mathies, Nat. Chem., 2018, 10, 449–455.
M. Gueye, M. Manathunga, D. Agathangelou, Y. Orozco, M. Paolino, S. Fusi, S. Haacke, M. Olivucci and J. Léonard, Nat. Commun., 2018, 9, 313.
I. Schapiro, S. Fusi, M. Olivucci, T. Andruniów, S. Sasidharanpillai and G. R. Loppnow, J. Phys. Chem. B, 2014, 118, 12243–12250.
R. Rossi Paccani, D. Donati, S. Fusi, L. Latterini, G. Farina, V. Zanirato and M. Olivucci, J. Org. Chem., 2012, 77, 1738–1748.
A. D. Dunkelberger, R. D. Kieda, J. Y. Shin, R. Rossi Paccani, S. Fusi, M. Olivucci and F. Fleming Crim, J. Phys. Chem. A, 2012, 116, 3527–3533.
J. Briand, O. Braem, J. Rehault, J. Léonard, A. Cannizzo, M. Chergui, V. Zanirato, M. Olivucci, J. Helbing and S. Haacke, Phys. Chem. Chem. Phys., 2010, 12, 3178–3187.
J. Léonard, I. Schapiro, J. Briand, S. Fusi, R. R. Paccani, M. Olivucci and S. Haacke, Chem. – Eur. J., 2012, 18, 15296–15304.
K. Pagano, M. Paolino, S. Fusi, V. Zanirato, C. Trapella, G. Giuliani, A. Cappelli, S. Zanzoni, H. Molinari, L. Ragona and M. Olivucci, J. Phys. Chem. Lett., 2019, 10, 2235–2243.
S. Gozem, F. Melaccio, H. L. Luk, S. Rinaldi and M. Olivucci, Chem. Soc. Rev., 2014, 43, 4019–4036.
A. Nikiforov, J. A. Gamez, W. Thiel and M. Filatov, J. Phys. Chem. Lett., 2016, 7, 105–110.
J. Wang and B. Durbeej, ChemistryOpen, 2018, 7, 583–589.
V. Zanirato, G. P. Pollini, C. De Risi, F. Valente, A. Melloni, S. Fusi, J. Barbetti and M. Olivucci, Tetrahedron, 2007, 63, 4975–4982.
G. Marchand, J. Eng, I. Schapiro, A. Valentini, L. M. Frutos, E. Pieri, M. Olivucci, J. Léonard and E. Gindensperger, J. Phys. Chem. Lett., 2015, 6, 599–604.
E. Alcalde, N. Mesquida, S. López-Pérez, J. Frigola, R. Mercè, J. Holenz, M. Pujol and E. Hernández, Bioorg. Med. Chem., 2009, 17, 7387–7397.
W. Wild, A. Seilmeier, N. H. Gottfried and W. Kaiser, Chem. Phys. Lett., 1985, 119, 259–263.
T. Robl and A. Seilmeier, Chem. Phys. Lett., 1988, 147, 544–550.
M. Gueye, M. Paolino, E. Gindensperger, S. Haacke, M. Olivucci and J. Léonard, Faraday Discuss., DOI: 10.1039/C9FD00062C.
A. Muñoz Losa, I. F. Galván, M. E. Martín and M. A. Aguilar, J. Phys. Chem. B, 2006, 110, 18064–18071.
M. E. Martín, A. M. Losa, I. F. Galván and M. A. Aguilar, J. Mol. Struct.: THEOCHEM, 2006, 775, 81–86.
A. Melloni, R. Rossi Paccani, D. Donati, V. Zanirato, A. Sinicropi, M. L. Parisi, E. Martin, M. Ryazantsev, W. J. Ding, L. M. Frutos, R. Basosi, S. Fusi, L. Latterini, N. Ferré and M. Olivucci, J. Am. Chem. Soc., 2010, 132, 9310–9319.
M. Klok, N. Boyle, M. T. Pryce, A. Meetsma, W. R. Browne and B. L. Feringa, J. Am. Chem. Soc., 2008, 130, 10484–10485.
A. Gerwien, P. Mayer and H. Dube, J. Am. Chem. Soc., 2018, 140, 16442–16445.
M. Paolino, M. Gueye, E. Pieri, M. Manathunga, S. Fusi, A. Cappelli, L. Latterini, D. Pannacci, M. Filatov, J. Léonard and M. Olivucci, J. Am. Chem. Soc., 2016, 138, 9807–9825.
C. I. Bayly, P. Cieplak, W. Cornell and P. A. Kollman, J. Phys. Chem., 1993, 97, 10269–10280.
W. D. Cornell, P. Cieplak, C. I. Bayly, I. R. Gould, K. M. Merz, D. M. Ferguson, D. C. Spellmeyer, T. Fox, J. W. Caldwell and P. A. Kollman, J. Am. Chem. Soc., 1995, 117, 5179–5197.
I. F. Galván, M. L. Sánchez, M. E. Martín, F. J. O. del Valle and M. A. Aguilar, Comput. Phys. Commun., 2003, 155, 244–259.
N. Ferré and J. G. Ángyán, Chem. Phys. Lett., 2002, 356, 331–339.
F. Aquilante, L. De Vico, N. Ferré, G. Ghigo, P. Å. Malmqvist, P. Neogrády, T. B. Pedersen, M. PitoNák, M. Reiher, B. O. Roos, L. Serrano, M. Urban, V. Veryazov and R. Lindh, J. Comput. Chem., 2010, 31, 224–247.
J. W. Ponder and F. M. Richards, J. Comput. Chem., 1987, 8, 1016–1024.
F. Melaccio, M. Olivucci, R. Lindh and N. Ferré, Int. J. Quantum Chem., 2011, 111, 3339–3346.
M. Manathunga, et al., J. Chem. Theory Comput., 2016, 12, 839–850.
J. G. Ángyán, J. Math. Chem., 1992, 10, 93–137.
A. Warshel and M. Levitt, J. Mol. Biol., 1976, 103, 227–249.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Professor Ugo Mazzucato of the University of Perugia, Italy.
Electronic supplementary information (ESI) available: Two trajectory movies (echiral.006.md.xyz and zchiral.004.md.xyz). See DOI: 10.1039/c9pp00223e
These authors have equally contributed to the research.
Rights and permissions
About this article
Cite this article
Schapiro, I., Gueye, M., Paolino, M. et al. Synthesis, spectroscopy and QM/MM simulations of a biomimetic ultrafast light-driven molecular motor. Photochem Photobiol Sci 18, 2259–2269 (2019). https://doi.org/10.1039/c9pp00223e
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c9pp00223e