Abstract
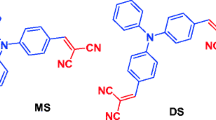
The one-photon (1P) and two-photon (2P) absorption properties of three quadrupolar dyes, featuring thiophene as a donor and acceptors of varying strengths, are determined by a combination of experimental and computational methods employing the density functional theory (DFT). The emission shifts in different solvents are well reproduced by time-dependent DFT calculations with the linear response and state specific approaches in the framework of the polarizable continuum model. The calculations show that the energies of both 1P- and 2P-active states decrease with an increase of the strength of the acceptor. The 2P absorption cross-sections predicted by the response theory are accounted for by considering just one intermediate state (S1) in the sum-over-states formulation. For the chromophore featuring the stronger acceptor, the energetic positions of the 1P- and 2P-active states prevent the exploitation of the theoretically predicted very high 2P activity due to the competing 1P absorption into the S1 state.
Similar content being viewed by others
References
Z. Guo, S. Park, J. Yoon and I. Shin, Recent progress in the development of near-infrared fluorescent probes for bioimaging applications, Chem. Soc. Rev., 2014, 43, 16.
E. A. Owens, M. Henary, G. El Fakhri and H. S. Choi, Tissue-Specific Near-Infrared Fluorescence Imaging, Acc. Chem. Res., 2016, 49, 1731.
E. Hemmer, A. Benayas, F. Légaré and F. Vetrone, Exploiting the biological windows: current perspectives on fluorescent bioprobes emitting above 1000 nm, Nanoscale Horiz., 2016, 1, 168–184.
T. Terai and T. Nagano, Small-molecule fluorophores and fluorescent probes for bioimaging, Pflügers Arch., 2013, 465, 347–359.
L. E. McNamara, N. Liyanage, A. Peddapuram, J. S. Murphy, J. H. Delcamp and N. I. Hammer, Donor-Acceptor-Donor Thienopyrazines via Pd-Catalyzed C-H Activation as NIR Fluorescent Materials, J. Org. Chem., 2016, 81, 32–42.
B. Zhou, Z. Hu, Y. Jiang, C. Zhon, Z. Sun and H. Sun, Theoretical exploitation of acceptors based on benzobis(thiadiazole) and derivatives for organic NIR-II fluorophores, Phys. Chem. Chem. Phys., 2018, 20, 19759–19767.
F. Di Maria, M. Biasiucci, F. P. Di Nicola, E. Fabiano, A. Zanelli, M. Gazzano, E. Salatelli, M. Lanzi, F. Della Sala, G. Gigli and G. Barbarella, Nanoscale Characterization and Unexpected Photovoltaic Behavior of Low Band Gap Sulfur-Overrich-Thiophene/Benzothiadiazole Decamers and Polymers, J. Phys. Chem. C, 2015, 119, 27200–27211.
N. Ghofraniha, I. Viola, F. Di Maria, G. Barbarella, G. Gigli and C. Conti, Random laser from engineered nanostructures obtained by surface tension driven lithography, Laser Photonics Rev., 2013, 7, 432–438.
F. Di Maria, M. Zangoli, I. E. Palamá, E. Fabiano, A. Zanelli, M. Monari, A. Perinot, M. Caironi, V. Maiorano, A. Maggiore, M. Pugliese, E. Salatelli, G. Gigli, I. Viola and G. Barbarella, Improving the Property–Function Tuning Range of Thiophene Materials via Facile Synthesis of Oligo/Polythiophene-S-Oxides and Mixed Oligo/Polythiophene-S-Oxides/Oligo/Polythiophene-S,S-Dioxides, Adv. Funct. Mater., 2016, 26, 6970–6984.
F. Di Maria, I. E. Palamà, M. Baroncini, A. Barbieri, A. Bongini, R. Bizzarri, G. Gigli and G. Barbarella, Live cell cytoplasm staining and selective labeling of intracellular proteins by non-toxic cell-permeant thiophene fluorophores, Org. Biomol. Chem., 2014, 12, 1603–1610.
M. Locritani, Y. Yu, G. Bergamini, J. K. Molloy, M. Baroncini, B. A. Korgel and P. Ceroni, Silicon nanocrystals functionalized with pyrene units: very efficient lightharvesting antennae with bright near-infrared emission, J. Phys. Chem. Lett., 2014, 5, 3325–3329.
R. Mazzaro, M. Locritani, J. K. Molloy, M. Montalti, Y. Yu, B. A. Korgel, G. Bergamini, V. Morandi and P. Ceroni, Photoinduced processes between pyrene-functionalized Silicon nanocrystals and carbon allotropes, Chem. Mater., 2015, 27 ,4390–4397.
A. Fermi, M. Locritani, G. Di Carlo, M. Pizzotti, S. Caramori, Y. Yu, B. A. Korgel, G. Bergamini and P. Ceroni, Light-harvesting antennae based on photoactive silicon nanocrystals functionalized with porphyrin chromophores, Faraday Discuss., 2015, 185, 481–495.
F. Romano, Y. Yu, B. A. Korgel, G. Bergamini and P. Ceroni, Light-harvesting antennae based on silicon nanocrystals, Top. Curr. Chem., 2016, 374, 53.
L. Ravotto, Q. Chen, Y. Ma, S. A. Vinogradov, M. Locritani, G. Bergamini, F. Negri, Y. Yu, B. A. Korgel and P. Ceroni, Bright Long-Lived Luminescence of Silicon Nanocrystals Sensitized by Two-Photon Absorbing Antenna, Chem, 2017, 2, 550–560.
M. Pawlicki, H. A. Collins, R. G. Denning and H. L. Anderson, Two-photon absorption and the design of two-photon dyes, Angew. Chem., Int. Ed., 2009, 48, 3244–3266.
Y. Zhang, S. A. Autry, L. E. McNamara, S. T. Nguyen, N. Le, P. Brogdon, D. L. Watkins, N. I. Hammer and J. H. Delcamp, Near-Infrared Fluorescent Thienothiadiazole Dyes with Large Stokes Shifts and High Photostability, J. Org. Chem., 2017, 82, 5597–5606.
S. I. Kato, T. Matsumoto, T. Ishi-i, T. Thiemann, M. Shigeiwa, H. Gorohmaru, S. Maeda, Y. Yamashita and S. Mataka, Strongly red-fluorescent novel donor–π-bridge–acceptor–π-bridge–donor (D–π–A–π–D) type 21, 3-benzothiadiazoles with enhanced two-photon absorption cross-sections, Chem. Commun., 2004, 2342–2343.
S. I. Kato, T. Matsumoto, M. Shigeiwa, H. Gorohmaru, S. Maeda, T. Ishi-I and S. Mataka, Novel 2, 1, 3-Benzothiadiazole-Based Red-Fluorescent Dyes with Enhanced Two-Photon Absorption Cross-Sections, Chem. – Eur. J., 2006, 12, 2303–2317.
S. Ellinger, K. R. Graham, P. Shi, R. T. Farley, T. T. Steckler, R. N. Brookins, P. Taranekar, J. Mei, L. A. Padilha, T. R. Ensley, H. Hu, S. Webster, D. J. Hagan, E. W. Van Stryland, K. S. Schanze and J. R. Reynolds, Donor-Acceptor-Donor-based π-Conjugated Oligomers for Nonlinear Optics and Near-IR Emission, Chem. Mater., 2011, 23, 3805–3817.
S. Yao, B. Kim, X. Yue, M. Y. Colon Gomez, M. V. Bondar and K. D. Belfield, Synthesis of Near-Infrared Fluorescent Two-Photon-Absorbing Fluorenyl Benzothiadiazole and Benzoselenadiazole Derivatives, ACS Omega, 2016, 1, 1149–1156.
T. Yanai, D. P. Tew and N. C. Handy, A new hybrid exchange–correlation functional using the Coulombattenuating method (CAM-B3LYP), Chem. Phys. Lett., 2004, 393, 51–57.
R. Cammi, S. Corni, B. Mennucci and J. Tomasi, Electronic excitation energies of molecules in solution: state specific and linear response methods for nonequilibrium continuum solvation models, J. Chem. Phys., 2005, 122, 104513.
S. Corni, R. Cammi, B. Mennucci and J. Tomasi, Electronic excitation energies of molecules in solution within continuum solvation models: investigating the discrepancy between state specific and linear response methods, J. Chem. Phys., 2005, 123, 134512.
M. Cossi and V. Barone, Time-dependent density functional theory for molecules in liquid solutions, J. Chem. Phys., 2001, 115, 4708–4717.
R. Cammi and B. Mennucci, Linear response theory for the polarizable continuum model, J. Chem. Phys., 1999, 110, 9877–9886.
R. Improta, G. Scalmani, M. J. Frisch and V. Barone, Toward effective and reliable fluorescence energies in solution by a newstate specific polarizable continuum model time dependent density functional theory approach, J. Chem. Phys., 2007, 127, 074504.
R. Improta, V. Barone, G. Scalmani and M. J. Frisch, A state-specific polarizable continuum model time dependent density functional theory method for excited state calculations in solution, J. Chem. Phys., 2006, 125, 054103.
F. Negri and M. Z. Zgierski, Franck–Condon analysis of the S0 → T1 absorption and phosphorescence spectra of biphenyl and bridged derivatives, J. Chem. Phys., 1992, 97, 7124.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford CT, 2009.
(a) D. H. Friese, M. T. P. Beerepoot, M. Ringholm and K. Ruud, Open-Ended Recursive Approach for the Calculation of Multiphoton Absorption Matrix Elements, J. Chem. Theory Comput., 2015, 11, 1129–1144; (b) M. T. P. Beerepoot, D. H. Friese, N. H. List, J. Kongsted and K. Ruud, Benchmarking two-photon absorption cross sections: performance of CC2 and CAM-B3LYP, Phys. Chem. Chem. Phys., 2015, 17, 19306–19314.
D. H. Friese, C. Hättig and K. Ruud, Calculation of two-photon absorption strengths with the approximate coupled cluster singles and doubles model CC2 using the resolution-of-identity approximation, Phys. Chem. Chem. Phys., 2012, 14, 1175–1184.
Dalton, a molecular electronic structure program, Release Dalton 2016.1 (2016), see http://daltonprogram.org.
K. Aidas, C. Angeli, K. L. Bak, V. Bakken, R. Bast, L. Boman, O. Christiansen, R. Cimiraglia, S. Coriani, P. Dahle, E. K. Dalskov, U. Ekström, T. Enevoldsen, J. J. Eriksen, P. Ettenhuber, B. Fernández, L. Ferrighi, H. Fliegl, L. Frediani, K. Hald, A. Halkier, C. Hättig, H. Heiberg, T. Helgaker, A. C. Hennum, H. Hettema, E. Hjertenæs, S. Høst, I.-M. Høyvik, M. F. Iozzi, B. Jansik, H. J. Aa. Jensen, D. Jonsson, P. Jørgensen, J. Kauczor, S. Kirpekar, T. Kjærgaard, W. Klopper, S. Knecht, R. Kobayashi, H. Koch, J. Kongsted, A. Krapp, K. Kristensen, A. Ligabue, O. B. Lutnæs, J. I. Melo, K. V. Mikkelsen, R. H. Myhre, C. Neiss, C. B. Nielsen, P. Norman, J. Olsen, J. M. H. Olsen, A. Osted, M. J. Packer, F. Pawlowski, T. B. Pedersen, P. F. Provasi, S. Reine, Z. Rinkevicius, T. A. Ruden, K. Ruud, V. Rybkin, P. Salek, C. C. M. Samson, A. Sánchez de Merás, T. Saue, S. P. A. Sauer, B. Schimmelpfennig, K. Sneskov, A. H. Steindal, K. O. Sylvester-Hvid, P. R. Taylor, A. M. Teale, E. I. Tellgren, D. P. Tew, A. J. Thorvaldsen, L. Thøgersen, O. Vahtras, M. A. Watson, D. J. D. Wilson, M. Ziolkowski and H. Ågren, The Dalton quantum chemistry program system, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2014, 4, 269–284.
S. Sanyal, A. Painelli, S. K. Pati, F. Terenziani and C. Sissa, Aggregates of quadrupolar dyes for two-photon absorption: the role of intermolecular interactions, Phys. Chem. Chem. Phys., 2016, 18, 28198.
I. Palamà, F. Di Maria, I. Viola, E. Fabiano, G. Gigli, C. Bettini and G. Barbarella, Live-Cell-Permeant Thiophene Fluorophores and Cell-Mediated Formation of Fluorescent Fibrils, J. Am. Chem. Soc., 2011, 133, 17777–17785.
C. Kitamura, S. Tanaka and Y. Yamashita, Synthesis of new narrow bandgap polymers based on 5,7-di(2-thienyl)thieno [3,4-b]pyrazine and its derivatives, J. Chem. Soc., Chem. Commun., 1994, 1585–1586.
I. Kmnek, D. Vyprachticky, J. Kriz, J. Dybal and V. Cimrova, Low-Band Gap Copolymers Containing Thienothiadiazole Units: Synthesis, Optical, and Electrochemical Properties, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 2743–2756.
G. A. Crosby and J. N. Demas, Measurement of photoluminescence quantum yields. Review, J. Phys. Chem., 1971, 75, 991–1024.
K. Suzuki, A. Kobayashi, S. Kaneko, K. Takehira, T. Yoshihara, H. Ishida, Y. Shiina, S. Oishi and S. Tobita, Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector, Phys. Chem. Chem. Phys., 2009, 11, 9850–9860.
C. Würth, M. Grabolle, J. Pauli, M. Spieles and U. Resch-Genger, Relative and absolute determination of fluorescence quantum yields of transparent samples, Nat. Protoc., 2013, 8, 1535–1550.
C. Würth, J. Pauli, C. Lochmann, M. Spieles and U. Resch-Genger, Integrating Sphere Setup for the Traceable Measurement of Absolute Photoluminescence Quantum Yields in the Near Infrared, Anal. Chem., 2012, 84, 1345–1352.
(a) C. Xu and W. W. Webb, Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm, J. Opt. Soc. Am. B, 1996, 13, 481; (b) N. S. Makarov, M. Drobizhev and A. Rebane, Two-photon absorption standards in the 550–1600 nm excitation wavelength range, Opt. Express, 2008, 16, 4029–4047.
A. Iagatti, B. Patrizi, A. Basagni, A. Marcelli, A. Alessi, S. Zanardi, R. Fusco, M. Salvalaggio, L. Bussotti and P. Foggi, Photophysical properties and excited state dynamics of 4,7-dithien-2-yl-2,1,3-benzothiadiazole, Phys. Chem. Chem. Phys., 2017, 19, 13604.
Y. Zhen, W. Yue, Y. Li, W. Jiang, S. Di Motta, E. Di Donato, F. Negri, S. Yea and Z. Wang, Chiral nanoribbons based on doubly-linked oligo-perylene bisimides, Chem. Commun., 2010, 46, 6078–6080.
D. Jacquemin, V. Wathelet, E. A. Perpte and C. Adamo, Extensive TD-DFT Benchmark: Singlet-Excited States of Organic Molecules, J. Chem. Theory Comput., 2009, 5, 2420–2435.
F. Santoro, V. Barone and R. Improta, Can TD-DFT calculations accurately describe the excited states behavior of stacked nucleobases? The cytosine dimer as a test case, J. Comput. Chem., 2008, 29, 957–964.
W. Jiang, C. Xiao, L. Hao, Z. Wang, H. Ceymann, C. Lambert, S. Di Motta and F. Negri, Localization/Delocalization of Charges in Bay-Linked Perylene Bisimides, Chem. – Eur. J., 2012, 18, 6764–6775.
D. Jacquemin, A. Planchat, C. Adamo and B. Mennucci, TD-DFT, Assessment of Functionals for Optical 0–0 Transitions in Solvated Dyes, J. Chem. Theory Comput., 2012, 8, 2359–2372.
A. Prlj, B. F. E. Curchod, A. Fabrizio, L. Floryan and C. Corminboeuf, Qualitatively incorrect features in the TDDFT spectrum of tiophene-based compounds, J. Phys. Chem. Lett., 2015, 6, 13–21.
A. Prlj, M. E. Sandoval-Salinas, D. Casanova, D. Jacquemin and C. Corminboeuf, Low-lying ππ* states of heteroaromatic molecules: a challenge for excited state methods, J. Chem. Theory Comput., 2016, 12, 2652–2660.
O. Uranga-Barandiaran, M. Catherin, E. Zaborova, A. D’Aléo, F. Fages, F. Castet and D. Casanova, Optical properties of quadrupolar and bi-quadrupolar dyes: intra and inter chromophoric interactions, Phys. Chem. Chem. Phys., 2018, 20, 24623–24632.
W. J. Meath and E. A. Power, On the importance of permanent moments in multiphoton absorption using perturbation theory, J. Phys. B: At. Mol. Phys., 1984, 17, 763–781.
K. Kristensen, J. Kauczor, A. J. Thorvaldsen, P. Jørgensen, T. Kjærgaard and A. Rizzo, Damped response theory description of two-photon absorption, J. Chem. Phys., 2011, 134, 214104.
T. V. Esipova, H. J. Rivera-Jacquez, B. Weber, A. E. Masunov and S. A. Vinogradov, Two-photon absorbing phosphorescent metalloporphyrins: effects of p-extension and peripheral substitution, J. Am. Chem. Soc., 2016, 138, 15648–15662.
M. Plidschun, M. Chemnitz and M. A. Schmidt, Low-loss deuterated organic solvents for visible and near-infrared photonics, Opt. Mater. Express|, 2017, 7, 1122–1130.
M. Drobizhev, A. Karotki, M. Kruk, A. Krivokapi, H. L. Anderson and A. Rebane, Photon energy upconversion in porphyrins: one-photon hot-band absorption versus two-photon absorption, Chem. Phys. Lett., 2003, 370, 690–699.
M. Nakano and B. Champagne, Theoretical Design of Open-Shell Singlet Molecular Systems for Nonlinear Optics, J. Phys. Chem. Lett., 2015, 6, 3236–3256.
M. Nakano, Open-Shell-Character-Based Molecular Design Principles: Applications to Nonlinear Optics and Singlet Fission, Chem. Rec., 2017, 17, 27–62.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available: Figures and tables with additional details on experimental and computational results. See DOI: 10.1039/c9pp00006b
Rights and permissions
About this article
Cite this article
Canola, S., Mardegan, L., Bergamini, G. et al. One- and two-photon absorption properties of quadrupolar thiophene-based dyes with acceptors of varying strengths. Photochem Photobiol Sci 18, 2180–2190 (2019). https://doi.org/10.1039/c9pp00006b
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c9pp00006b