Abstract
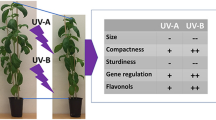
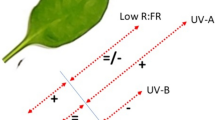
The UVR8 photoreceptor in Arabidopsis thaliana is specific for ultraviolet-B (UV-B; 280–315 nm) radiation and its activation leads to a number of UV-B acclimation responses, including the accumulation of flavonoids. UVR8 participates in a signaling cascade involving COP1 and HY5 so that the absence of any of these components results in a reduction in the ability of a plant to accumulate flavonoids in response to UV; Cop1 mutants show high dropouts and hy5-ks50 hyh double mutants show very low levels of flavonoids. The predominant phenolics in Arabidopsis thaliana are sinapic acid derivatives as well as non-aclyated quercetin and kaempferol di- and triglycosides containing glucose and rhamnose as glycosylated sugar moieties. How this flavonoid profile in Arabidopsis thaliana is affected by UV radiation, how rapidly these changes occur in changing UV conditions, and which components of the UV-B signalling pathway are involved in rapid UV acclimatization reactions is poorly understood. In the present study, we examined these questions by characterizing the flavonoid profiles of Arabidopsis thaliana signalling mutants and wild types grown under different UV levels of constant UV-B+PAR ratios and then transferring a subset of plants to alternate UV conditions. Results indicate that flavonoid accumulation in Arabidopsis thaliana is triggered by UV and this response is amplified by higher levels of UV but not by all compounds to the same extent. The catechol structure in quercetin seems to be less important than the glycosylation pattern, e.g. having 2 rhamnose moieties in determining responsivity. At low UV+PAR intensities the introduction of UV leads to an initial tendency of increase of flavonoids in the wild types that was detected after 3 days. It took 7 days for these changes to be detected in plants grown under high UV+PAR intensities suggesting a priming of PAR. Thus, the flavonoid profile in Arabidopsis thaliana is altered over time following exposure to UV and PAR, but the functional significance of these changes is currently unclear.
Similar content being viewed by others
References
M. A. K. Jansen and J. F. Bornman, Physiol. Plant., 2012, 145(4), 501.
D. Verdaguer, M. A. K. Jansen, L. Llorens, L. O. Morales and S. Neugart, Plant Sci., 2017, 255(Suppl. C), 72.
B. R. Jordan, Funct. Plant Biol., 2002, 29(8), 909.
K. R. Albert, T. N. Mikkelsen, H. Ro-Poulsen, M. F. Arndal and A. Michelsen, Environ. Exp. Bot., 2011, 73, 10.
E. Hideg, M. A. K. Jansen and A. Strid, Trends Plant Sci., 2013, 18(2), 107.
M. Heijde and R. Ulm, Trends Plant Sci., 2012, 17(4), 230.
G. I. Jenkins, The Plant Cell, 2014, 26(1), 21.
A. B. Britt, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1996, 47, 75.
M. M. Caldwell, R. Robberecht and S. D. Flint, Physiol. Plant., 1983, 58(3), 445.
L. G. Landry, C. Chapple and R. L. Last, Plant Physiol., 1995, 109(4), 1159.
G. Agati, C. Brunetti, M. Di Ferdinando, F. Ferrini, S. Pollastri and M. Tattini, Plant Physiol. Biochem., 2013, 72, 35.
L. Rizzini, J.-J. Favory, C. Cloix, D. Faggionato, A. O’Hara, E. Kaiserli, R. Baumeister, E. Schäfer, F. Nagy, G. I. Jenkins and R. Ulm, Science, 2011, 332(6025), 103.
L. O. Morales, M. Brosche, J. Vainonen, G. I. Jenkins, J. J. Wargent, N. Sipari, A. Strid, A. V. Lindfors, R. Tegelberg and P. J. Aphalo, Plant Physiol., 2013, 161(2), 744.
C. Lang-Mladek, L. S. Xie, N. Nigam, N. Chumak, M. Binkert, S. Neubert and M. T. Hauser, Physiol. Plant., 2012, 145(4), 527.
B. A. Brown, C. Cloix, G. H. Jiang, E. Kaiserli, P. Herzyk, D. J. Kliebenstein and G. I. Jenkins, Proc. Natl. Acad. Sci. U. S. A., 2005, 102(50), 18225.
J. J. Favory, A. Stec, H. Gruber, L. Rizzini, A. Oravecz, M. Funk, A. Albert, C. Cloix, G. I. Jenkins, E. J. Oakeley, H. K. Seidlitz, F. Nagy and R. Ulm, EMBO J., 2009, 28(5), 591.
G. Agati and M. Tattini, New Phytol., 2010, 186(4), 786.
K. Saito, K. Yonekura-Sakakibara, R. Nakabayashi, Y. Higashi, M. Yamazaki, T. Tohge and A. R. Fernie, Plant Physiol. Biochem., 2013, 72, 21.
M. A. K. Jansen, K. Hectors, N. M. O’Brien, Y. Guisez and G. Potters, Plant Sci., 2008, 175(4), 449.
P. Majer, S. Neugart, A. Krumbein, M. Schreiner and É. Hideg, Environ. Exp. Bot., 2014, 100, 1.
M. Fiol, S. Adermann, S. Neugart, S. Rohn, C. Mügge, M. Schreiner, A. Krumbein and L. W. Kroh, Food Res. Int., 2012, 47(1), 80.
L. C. Olsson, M. Veit, G. Weissenbock and J. F. Bornman, Phytochemistry, 1998, 49(4), 1021.
B. Harbaum-Piayda, B. Walter, G. B. Bengtsson, E. M. Hubbermann, W. Bilger and K. Schwarz, Postharvest Biol. Biotechnol., 2010, 56(3), 202.
S. Neugart, M. Zietz, M. Schreiner, S. Rohn, L. W. Kroh and A. Krumbein, Physiol. Plant., 2012, 145(4), 582.
S. Neugart, M. Fiol, M. Schreiner, S. Rohn, R. Zrenner, L. W. Kroh and A. Krumbein, J. Agric. Food Chem., 2014, 62(18), 4054.
C. M. M. Gachon, M. Langlois-Meurinne and P. Saindrenan, Trends Plant Sci., 2005, 10(11), 542.
D. Bowles, E. K. Lim, B. Poppenberger and F. E. Vaistij, Annu. Rev. Plant Biol., 2006, 57, 567.
O. Rechner, S. Neugart, M. Schreiner, S. Wu and H. M. Poehling, J. Chem. Ecol., 2016, 42(10), 989.
M. Moreira-Rodríguez, V. Nair, J. Benavides, L. Cisneros-Zevallos and D. A. Jacobo-Velázquez, Molecules, 2017, 22, 1065.
A. Edreva, Agric., Ecosyst. Environ., 2005, 106(2–3), 135.
I. Hernandez, L. Alegre, F. van Breusegem and S. Munne-Bosch, Trends Plant Sci., 2009, 14(3), 125.
C. A. Mazza, H. E. Boccalandro, C. V. Giordano, D. Battista, A. L. Scopel and C. L. Ballare, Plant Physiol., 2000, 122(1), 117.
L. P. R. Bidel, S. Meyer, Y. Goulas, Y. Cadot and Z. G. Cerovic, J. Photochem. Photobiol., B, 2007, 88(2), 163.
C. E. Williamson, R. G. Zepp, R. M. Lucas, S. Madronich, A. T. Austin, C. L. Ballaré, M. Norval, B. Sulzberger, A. F. Bais, R. L. McKenzie, S. A. Robinson, D.-P. Häder, N. D. Paul and J. F. Bornman, Nat. Clim. Change, 2014, 4, 434–446.
J. F. Bornman, P. W. Barnes, S. A. Robinson, C. L. Ballare, S. D. Flint and M. M. Caldwell, Photochem. Photobiol. Sci., 2015, 14(1), 88.
P. S. Searles, S. D. Flint and M. M. Caldwell, Oecologia, 2001, 127(1), 1.
P. W. Barnes, T. M. Robson, M. A. Tobler, I. N. Bottger and S. D. Flint, UV-B Radiation and Plant Life. Chapter Molecular Biology to Ecology, ed. B. Jordan, CABI International, 2017, p. 72.
M. Goetz, A. Albert, S. Stich, W. Heller, H. Scherb, A. Krins, C. Langebartels, H. K. Seidlitz and D. Ernst, Protoplasma, 2010, 243(1–4), 95.
G. I. Jenkins, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2009, 153A(2), S203–S203.
M. M. Caldwell, Solar UV irradiation and the growth and development of higher plants, Academic Press, New York, 1971.
J. Schindelin, I. Arganda-Carreras, E. Frise, V. Kaynig, M. Longair, T. Pietzsch, S. Preibisch, C. Rueden, S. Saalfeld, B. Schmid, J. Y. Tinevez, D. J. White, V. Hartenstein, K. Eliceiri, P. Tomancak and A. Cardona, Nat. Methods, 2012, 9(7), 676.
S. Neugart, S. Baldermann, B. Ngwene, J. Wesonga and M. Schreiner, Food Res. Int., 2017, 100, 411.
S. Schmidt, M. Zietz, M. Schreiner, S. Rohn, L. W. Kroh and A. Krumbein, Rapid Commun. Mass Spectrom., 2010, 24(14), 2009.
P. Majer and E. Hideg, Emir. J. Food Agric., 2012, 24(6), 598.
G. Agati, Z. G. Cerovic, P. Pinelli and M. Tattini, Environ. Exp. Bot., 2011, 73, 3.
P. W. Barnes, M. A. Tobler, K. Keefover-Ring, S. D. Flint, A. E. Barkley, R. J. Ryel and R. L. Lindroth, Plant, Cell Environ., 2016, 39(1), 222.
D. J. Kliebenstein, J. E. Lim, L. G. Landry and R. L. Last, Plant Physiol., 2002, 130(1), 234.
P. W. Barnes, A. R. Kersting, S. D. Flint, W. Beyschlag and R. J. Ryel, Physiol. Plant., 2013, 149(2), 200.
R. Yin, M. Y. Skvortsova, S. Loubery and R. Ulm, Proc. Natl. Acad. Sci. U. S. A., 2016, 113(30), E4415–E4422.
A. Oravecz, A. Baumann, Z. Máté, A. Brzezinska, J. Molinier, E. J. Oakeley, É. Ádám, E. Schäfer, F. Nagy and R. Ulm, Plant Cell, 2006, 18(8), 1975.
R. Stracke, J. J. Favory, H. Gruber, L. Bartelniewoehner, S. Bartels, M. Binkert, M. Funk, B. Weisshaar and R. Ulm, Plant, Cell Environ., 2010, 33(1), 88.
K. Tilbrook, A. B. Arongaus, M. Binkert, M. Heijde, R. Yin and R. Ulm, Arabidopsis Book, 2013, 11, e0164.
A. Gaudinier, M. Tang and D. J. Kliebenstein, Curr. Plant Biol., 2015, 3–4, 56.
Author information
Authors and Affiliations
Additional information
Electronic supplementary information (ESI) available. See DOI: 10.1039/c8pp00496j
Rights and permissions
About this article
Cite this article
Neugart, S., Tobler, M.A. & Barnes, P.W. Different irradiances of UV and PAR in the same ratios alter the flavonoid profiles of Arabidopsis thaliana wild types and UV-signalling pathway mutants. Photochem Photobiol Sci 18, 1685–1699 (2019). https://doi.org/10.1039/c8pp00496j
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c8pp00496j