Abstract
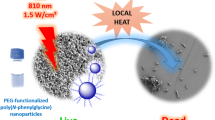
Here we report the preparation of water-dispersible glycosylated poly(2,5’-thienylene)porphyrin based nanoparticles by a nanoprecipitation method and demonstrate the application of these nanoparticles in antibacterial photodynamic therapy. The diameter of the nanoparticles is in the range of 50–80 nm and the resulting nanoparticles are stable in water without precipitation at least for a month. They have high singlet oxygen efficiency and display light-triggered biocidal activity against both Gram negative bacteria (Escherichia coli, E. coli) and Gram positive bacteria (Bacillus subtilis, B. subtilis). Upon white light irradiation for 10 min with a flux of 22 mW cm−2 of the E. coli suspension incubated with NPs (18 μg mL−1), a killing efficiency of 99% is achieved, whereas in the dark the effect is recorded as only around 8%.
Similar content being viewed by others
Notes and references
WHO, WHO Library Cataloguing-in-Publication Data, Antimicrobial resistance: global report on surveillance, 2014, pp. 1–232.
A. Fajardo, N. Martinez-Martin, M. Mercadillo, J. C. Galan, B. Ghysels, S. Matthijs, P. Cornelis, L. Wiehlmann, B. Tummler, F. Baquero and J. L. Martinez, The neglected intrinsic resistome of bacterial pathogens, PLoS One, 2008, 3, e1619.
T. Maisch, Resistance in antimicrobial photodynamic inactivation of bacteria, Photochem. Photobiol. Sci., 2015, 14, 1518–1526.
T. Maisch, A new strategy to destroy antibiotic resistant microorganisms: antimicrobial photodynamic treatment, Mini-Rev. Med. Chem., 2009, 9, 974–983.
E. C. Ziegelhoffer and T. J. Donohue, Bacterial responses to photo-oxidative stress, Nat. Rev. Microbiol., 2009, 7, 856–863.
D. Phillips, Light relief: photochemistry and medicine, Photochem. Photobiol. Sci., 2010, 9, 1589–1596.
B. C. Wilson and M. S. Patterson, The physics, biophysics and technology of photodynamic therapy, Phys. Med. Biol., 2008, 53, R61–R109.
J. S. Lindsey, Synthesis of meso-Substituted Porphyrins, in The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Academic Press, New York, 2000, vol. 1, pp. 67–118.
R. Bonnett, Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy, Chem. Soc. Rev., 1995, 24, 19–33.
E. D. Sternberg, D. Dolphin and C. Brückner, Porphyrinbased photosensitizers for use in photodynamic therapy, Tetrahedron, 1998, 54, 4151–4202.
S. Singh, A. Aggarwal, N. V. S. D. K. Bhupathiraju, G. Arianna, K. Tiwari and C. M. Drain, Glycosylated porphyrins, phthalocyanines, and other porphyrinoids for diagnostics and therapeutics, Chem. Rev., 2015, 115, 10261–10306.
Y. Wang, K. S. Schanze, E. Y. Chi and D. G. Whitten, When worlds collide: interactions at the interface between biological systems and synthetic cationic conjugated polyelectrolytes and oligomers, Langmuir, 2013, 29, 10635–10647.
C. Zhu, L. Liu, Q. Yang, F. Lv and S. Wang, Water-soluble conjugated polymers for imaging, diagnosis, and therapy, Chem. Rev., 2012, 112, 4687–4735.
H. Sun, B. Yin, H. Ma, H. Yuan, B. Fu and L. Liu, Synthesis of a novel quinoline skeleton introduced cationic polyfluorene derivative for multimodal antimicrobial application, ACS Appl. Mater. Interfaces, 2015, 7, 25390–25395.
Q. Zhao, J. Li, X. Zhang, Z. Li and Y. Tang, Cationic Oligo (thiophene ethynylene) with broad-spectrum and high antibacterial efficiency under white light and specific biocidal activity against S. aureus in dark, ACS Appl. Mater. Interfaces, 2016, 8, 1019–1024.
Y. Tang, T. S. Corbitt, A. Parthasarathy, Z. Zhou, K. S. Schanze and D. G. Whitten, Light-induced antibacterial activity of symmetrical and asymmetrical oligophenylene ethynylenes, Langmuir, 2011, 27, 4956–4962.
S. Li, K. Chang, K. Sun, Y. Tang, N. Cui, Y. Wang, W. Qin, H. Xu and C. Wu, Amplified singlet oxygen generation in semiconductor polymer dots for photodynamic cancer therapy, ACS Appl. Mater. Interfaces, 2016, 8, 3624–3634.
C. Xing, Q. Xu, H. Tang, L. Liu and S. Wang, Conjugated polymer/porphyrin complexes for efficient energy transfer and improving light-activated antibacterial activity, J. Am. Chem. Soc., 2009, 131, 13117–13124.
I. Yoon, J. Z. Li and Y. K. Shim, Advance in Photosensitizers and Light Delivery for Photodynamic Therapy, Clin. Endosc., 2013, 46(1), 7–23.
D. Ma, Z.-H. Liu, Q.-Q. Zheng, X.-Y. Zhou, Y. Zhang, Y.-F. Shi, J.-T. Lin and W. Xue, Star-shaped polymer consisting of a porphyrin core and poly (L-lysine) dendron arms: synthesis, drug delivery, and in vitro chemo/photodynamic therapy, Macromol. Rapid Commun., 2013, 34, 548–552.
L. Xu, L. Liu, F. Liu, W. Li, R. Chen, Y. Gao and W. Zhang, Photodynamic therapy of oligoethylene glycol dendronized reduction-sensitive porphyrins, J. Mater. Chem. B, 2015, 3, 3062–3071.
Y. Chen, D. Zhao and Y. Liu, Polysaccharide–porphyrin–fullerene supramolecular conjugates as photo-driven DNA cleavage reagents, Chem. Commun., 2015, 51, 12266–12269.
G. Garcia, D. Naud-Martin, D. Carrez, A. Croisy and P. Maillard, Microwave-mediated ‘click-chemistry’synthesis of glycoporphyrin derivatives and in vitro photocytotoxicity for application in photodynamic therapy, Tetrahedron, 2011, 67, 4924–4932.
S. Mandal, S. Bhattacharyya, V. Borovkov and A. Patra, Porphyrin-based functional nanoparticles: conformational and photophysical properties of bis-porphyrin and bis-porphyrin encapsulated polymer nanoparticles, J. Phys. Chem. C, 2011, 115, 24029–24036.
L. Zhao, R. Qu, A. Li, R. Mab and L. Shi, Cooperative self-assembly of porphyrins with polymers possessing bioactive functions, Chem. Commun., 2016, 52, 13543–13555.
X. Gong, T. Milic, C. Xu, J. D. Batteas and C. M. Drain, Preparation and characterization of porphyrin nanoparticles, J. Am. Chem. Soc., 2002, 124, 14290–14291.
H. Zhang, B. Zhang, M. Zhu, S. M. Grayson, R. Schmehl and J. Jayawickramarajah, Water-soluble porphyrin nanospheres: enhanced photo-physical properties achieved via cyclodextrin driven double self-inclusion, Chem. Commun., 2014, 50, 4853–4855.
J. Zhao, H.-Y. Zhang, H.-L. Sun and Y. Liu, Supramolecular nanoassemblies of an amphiphilic porphyrin–cyclodextrin conjugate and their morphological transition from vesicle to network, Chem. – Eur. J., 2015, 21, 4457–4464.
Y. Liu, T. Pauloehrl, S. I. Presolski, L. Albertazzi, A. R. A. Palmans and E. W. Meijer, Modular synthetic platform for the construction of functional single-chain polymeric nanoparticles: from aqueous catalysis to photosensitization, J. Am. Chem. Soc., 2015, 137, 13096–13105.
W.-D. Quan, A. Pitto-Barry, L. A. Baker, E. Stulz, R. Napier, R. K. O’Reilly and V. G. Stavros, Retaining individualities: the photodynamics of self-ordering porphyrin assemblies, Chem. Commun., 2016, 52, 1938–1941.
D. A. Roberts, M. J. Crossley and S. Perrier, Fluorescent bowl-shaped nanoparticles from ‘clicked’porphyrin–polymer conjugates, Polym. Chem., 2014, 5, 4016–4021.
D. A. Roberts, T. W. Schmidt, M. J. Crossley and S. Perrier, Tunable Self-Assembly of Triazole-Linked Porphyrin–Polymer Conjugates, Chem. – Eur. J., 2013, 19, 12759–12770.
B. Wang, H. Yuan, C. Zhu, Q. Yang, F. Lv, L. Liu and S. Wang, Polymer-drug conjugates for intracellular molecule-targeted photoinduced inactivation of protein and growth inhibition of cancer cells, Sci. Rep., 2012, 2, 766.
K. Liu, Y. Liu, Y. Yao, H. Yuan, S. Wang, Z. Wang and X. Zhang, Supramolecular photosensitizers with enhanced antibacterial efficiency, Angew. Chem., Int. Ed., 2013, 125, 8443–8447.
P. Mroz, J. Bhaumik, D. K. Dogutan, Z. Aly, Z. Kamal, L. Khalid, H. L. Kee, D. F. Bocian, D. Holten, J. S. Lindsey and M. R. Hamblin, Imidazole metalloporphyrins as photosensitizers for photodynamic therapy: Role of molecular charge, central metal and hydroxyl radical production, Cancer Lett., 2009, 282(1), 63–76.
R. Daly, G. Vaz, A. M. Davies, M. O. Senge and E. M. Scanlan, Synthesis and biological evaluation of a library of glycoporphyrin compounds, Chem. – Eur. J., 2012, 18, 14671–14679.
S. Silva, P. M. R. Pereira, P. Silva, F. A. A. Paz, M. A. F. Faustino, J. A. S. Cavaleiroa and J. P. C. Tome, Porphyrin and phthalocyanine glycodendritic conjugates: synthesis, photophysical and photochemical properties, Chem. Commun., 2012, 48, 3608–3610.
S. Vedachalam, B.-H. Choi, K. K. Pasunooti, K. M. Ching, K. Lee, H. S. Yoon and X.-W. Liu, Glycosylated porphyrin derivatives and their photodynamic activity in cancer cells, MedChemComm, 2011, 2, 371–377.
H.-R. Jia, Y.-X. Zhu, Z. Chen and F.-G. Wu, Cholesterolassisted bacterial cell surface engineering for photodynamic inactivation of Gram-positive and Gram-negative bacteria, ACS Appl. Mater. Interfaces, 2017, 9, 15943–15951.
R. Khan, M. Idris and D. Tuncel, Synthesis and investigation of singlet oxygen production efficiency of photosensitizers based on meso-phenyl-2,5-thienylene linked porphyrin oligomers and polymers, Org. Biomol. Chem., 2015, 13, 10496–10504.
N. Adarsh, R. R. Avirah and D. Ramaiah, Tuning photosensitized singlet oxygen generation efficiency of novel aza-BODIPY dyes, Org. Lett., 2010, 12, 5720–5723.
G. A. Pankuch, G. Lin, D. B. Hoellman, C. E. Good, M. R. Jacobs and P. C. Appelbaum, Activity of retapamulin against Streptococcus pyogenes and Staphylococcus aureus evaluated by agar dilution, microdilution, E-test, and disk diffusion methodologies, Antimicrob. Agents Chemother., 2006, 50, 1727–1730.
T. Gensch, C. Viappiani and S. E. Braslavsky, Structural volume changes upon photoexcitation of porphyrins: role of the nitrogen–water interactions, J. Am. Chem. Soc., 1999, 121, 10573–10582.
S. Banfi, E. Caruso, L. Buccafurni, V. Battini, S. Zazzaron, P. Barbieri and V. Orlandi, Antibacterial activity of tetraarylporphyrin photosensitizers: an in vitro study on Gram negative and Gram positive bacteria, J. Photochem. Photobiol., B, 2006, 85, 28–38.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available: Full synthetic scheme for PTTP-Glu-Ac, synthetic procedure for PTTP, 1H, 13C-NMR spectra of PTTP, 1H, 13C-NMR, FT-IR, UV-Vis, PL spectra of PTTP-Glu-Ac, time-dependent decrease of absorbance spectra for DPBF with NPs, minimum inhibitory concentration plots of NPs against E. coli in the dark and under light, plate photographs for NPs against B. subtilis on YTD agar plate in the dark and under light. See DOI: 10.1039/c8pp00470f
These authors contributed equally.
Rights and permissions
About this article
Cite this article
Khan, R., Özkan, M., Khaligh, A. et al. Water-dispersible glycosylated poly (2,5’-thienylene)porphyrin-based nanoparticles for antibacterial photodynamic therapy. Photochem Photobiol Sci 18, 1147–1155 (2019). https://doi.org/10.1039/c8pp00470f
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c8pp00470f