Abstract
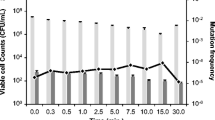
This study presents the first report on the photocatalytic inactivation mechanism for a Salmonella typhimurium pathogen by visible-light active CuxO loaded rhodium–antimony co-doped TiO2 nanorods (CuxO/Rh–Sb–TiO2 NRs) under visible light irradiation (cutoff filter, λ ≥ 420 nm). Remarkably higher pathogenic inactivation of 4 log within 40 min by a CuxO supported Rh–Sb–TiO2 NR photocatalyst was observed. The visible light active photocatalyst mainly produced reduced Cu+ in the lattice of CuxO by charge separation. By this means, photo-generated electrons at the conduction band of Rh–Sb–TiO2 NRs play an important role in reducing Cu2+ to Cu+ through the photocatalytic reduction reaction (PRR), and at the valence band of Rh–Sb–TiO2 NRs, photo-generated holes generate OH• radicals through the photocatalytic oxidation reaction (POR). This Cu+ copper species is lethal to microbial pathogens. The inactivation mechanism for the Salmonella typhimurium pathogen was investigated by protein oxidation, HCHO production, and the API-ZYM system. To investigate the role of OH• radicals, t-BuOH and MeOH as hole scavengers were used in photocatalytic inactivation reactions. Our experimental results confirmed that the reduced Cu+ species play a major role in bacterial inactivation, while ROS have a major effect on the degradation of organic pollutants.
Similar content being viewed by others
References
L. Ruggieri, E. Cadena, J. Martínez-Blanco, C. M. Gasol, J. Rieradevall, X. Gabarrell, T. X. Gea Sort and A. Sánchez, Recovery of organic wastes in the spanish wine industry, technical, economic and environmental analyses of the composting process, J. Cleaner Prod., 2009, 17(9), 830–838.
M. S. Lucas, J. A. Peres and G. Li Puma, Treatment of winery wastewater by ozone-based advanced oxidation processes (O3, O3/UV and O3/UV/H2O2) in a pilot-scale bubble column reactor and process economics, Sep. Purif. Technol., 2010, 72(3), 235–241.
J. C. Ireland, P. Klostermann, E. W. Rice and R. M. Clark, Inactivation of E.coli by titanium dioxide photocatalytic oxidation, Appl. Environ. Microbiol., 1993, 59(5), 1668–1670.
A. R. Khataee and M. B. Kasiri, Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: influence of the chemical structure of dyes, J. Mol. Catal. A: Chem., 2010, 328, 8–26.
Md. B. Ahmed, J. L. Zhou, H. H. Ngo, W. Guo, N. S. Thomaidis and J. Xu, Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A Critical Review, J. Hazard. Mater., 2017, 323A(5), 274–298.
T. Aarthi, P. Narahari and G. Madras, Photocatalytic degradation of azure and sudan dyes using nano TiO2, J. Hazard. Mater., 2007, 149(3), 725–734.
L. Rizzo, Inactivation and injury of total coliform bacteria after primary disinfection of drinking water by TiO2, J. Hazard. Mater., 2009, 165(1–3), 48–51.
J. Kuncewicz and B. Ohtani, Titania photocatalysis through two-photon band-gap excitation with built-in rhodium redox mediator, Chem. Commun., 2015, 51(2), 298–301.
F. E. Oropeza and R. G. Egdell, Control of valence states in Rh-doped TiO2 by Sb Co-doping: A study by high resolution X-ray photoemission spectroscopy, Chem. Phys. Lett., 2011, 515(4–6), 249–253.
R. Niishiro, R. Konta, H. Kato, W. J. Chun, K. Asakura and A. Kudo, Photocatalytic O−2 evolution of rhodium and antimony-codoped rutile-type TiO2 under visible light irradiation, J. Phys. Chem. C, 2007, 111(46), 17420–17426.
L. C. Liu, Z. Y. Ji, W. X. Zou, X. R. Gu, Y. Deng, F. Gao, C. J. Tang and L. Dong, In situ loading transition metal oxide clusters on TiO2 nanosheets as co-catalysts for exceptional high photoactivity, ACS Catal., 2013, 3(9), 2052–2061.
L. K. Dhandole, M. A. Mahadik, S. G. Kim, H. S. Chung, Y. S. Seo, M. Cho, J. Ryu and J. S. Jang, Boosting photocatalytic performance of inactive rutile TiO2 nanorods under solar light irradiation: synergistic effect of acid treatment and metal oxide co-catalysts, ACS Appl. Mater. Interfaces, 2017, 9(28), 23602–23613.
A. Di Paola, E. G. Lopez, G. Marci and L. Palmisano, A survey of photocatalytic materials for environmental remediation, J. Hazard. Mater., 2012, 211, 3–29.
M. Cho, H. M. Chung, W. Y. Choi and J. Y. Yoon, Different inactivation behaviors of MS-2 phage and escherichia Coli in TiO2 photocatalytic disinfection, Appl. Environ. Microbiol., 2005, 71(1), 270–275.
J. Shim, Y. S. Seo, B. T. Oh and M. Cho, Microbial inactivation kinetics and mechanisms of carbon-doped TiO2 (C-TiO2) under visible light, J. Hazard. Mater., 2016, 306, 133–139.
Q. Li, W. Liang and J. K. Shang, Enhanced visible-light absorption from PdO nanoparticles in nitrogen-doped titanium oxide thin films, Appl. Phys. Lett., 2007, 90(6), 0631091–0631093.
P. G. Wu, R. C. Xie, J. A. Imlay and J. K. Shang, Visiblelight-induced photocatalytic inactivation of bacteria by composite photocatalysts of palladium oxide and nitrogendoped titanium oxide, Appl. Catal., B, 2009, 88(3–6), 576–581.
L. K. Dhandole, S. Kim, Y. Seo, M. Mahadik, H. Chung, S. Lee, S. Choi, J. Ryu and J. Jang, Enhanced photocatalytic degradation of organic pollutants and inactivation of Listeria monocytogenes by visible light active Rh−Sb codoped TiO2 nanorods, ACS Sustainable Chem. Eng., 2018, 6, 4302–4315.
L. K. Dhandole, J. Ryu, J. M. Lim, B. T. Oh, J. H. Park, B. G. Kim and J. S. Jang, Hydrothermal synthesis of titanate nanotubes from TiO2 nanorods prepared via a molten salt flux method as an effective adsorbent for strontium ion recovery, RSC Adv., 2016, 6(100), 98449–98456.
G. J. Leclerc, C. Tartera and E. S. Metcalf, Environmental Regulation of Salmonella typhi Invasion-Defective Mutants, Infect. Immun., 1998, 66(2), 682–691.
R. Edelman and M. M. Levine, Summary of an International Workshop on Typhoid Fever, Rev. Infect. Dis., 1986, 8(3), 329–349.
M. Cho, H. Chung, W. Choi and J. Yoon, Linear correlation between inactivation of E-coli and OH radical concentration in TiO2 photocatalytic disinfection, Water Res., 2004, 38(4), 1069–1077.
M. Cho, J. Lee, Y. Mackeyev, L. J. Wilson, P. J. J. Alvarez, J. B. Hughes and J. H. Kim, Visible light sensitized inactivation of MS-2 bacteriophage by a cationic amine-functionalized C-60 derivative, Environ. Sci. Technol., 2010, 44(17), 6685–6691.
E. L. Cates, M. Cho and J. H. Kim, Converting visible light into UVC: microbial inactivation by Pr3+-activated upconversion materials, Environ. Sci. Technol., 2011, 45(8), 3680–3686.
Instruction manual for the protein carbonyl colorimetric assay kit from Cayman Chemical Co., item No. 10005020. https://www.caymanchem.com.
C. R. Keenan and D. L. Sedlak, Factors affecting the yield of oxidants from the reaction of nanoparticulate zero-valent iron and oxygen, Environ. Sci. Technol., 2008, 42(4), 1262–1267.
A. Chudnicka and G. Matysik, Research of enzymatic activities of fresh juice and water infusions from dry herbs, J. Ethnopharmacol., 2005, 99(2), 281–286.
M. Cho, J. Kim, J. Y. Kim, J. Kim and J. H. Kim, Mechanisms of Escherichia coli inactivation by several disinfectants, Water Res., 2010, 44, 3410–3418.
C. Hatchard and C. Parker, A new sensitive chemical actinometer II. potassium ferrioxalate as a standard chemical actinometer, Proc. R. Soc. London, Ser. A, 1956, 235, 518–536.
M. Cho, J. Fortner, J. Hughes and J. Kim, Escherichia coli inactivation by water-soluble, ozonated C60 derivative: kinetics and mechanisms, Environ. Sci. Technol., 2009, 43, 7410–7415.
T. Cooney, Bactericidal activity of copper and non-copper paints, Infect. Control Hosp. Epidemiol., 1995, 16(8), 444–450.
M. Paschoalino, N. C. Guedes, W. Jardim, E. Mieluarski, J. A. Mielczarski, P. Bowen and J. Kiwi, Inactivation of E-coli mediated by high surface area CuO accelerated by light irradiation > 360 nm, J. Photochem. Photobiol., A, 2008, 199(1), 105–111.
A. Torres, C. Ruales, C. Pulgarin, A. Aimable, P. Bowen, V. Sarria and J. Kiwi, Innovative high-surface-area CuO pretreated cotton effective in bacterial inactivation under visible light, ACS Appl. Mater. Interfaces, 2010, 2(9), 2547–2552.
S. Goldstein, G. Czapski and D. Meyerstein, A mechanistic study of the copper(II) peptide-catalyzed superoxide dismutation - a pulse-radiolysis study, J. Am. Chem. Soc., 1990, 112(18), 6489–6492.
H. J. Park, T. T. M. Nguyen, J. Yoon and C. Lee, Role of reactive oxygen species in escherichia coli inactivation by cupric ion, Environ. Sci. Technol., 2012, 46(20), 11299–11304.
J. T. Lisle, B. H. Pyle and G. A. McFeters, The use of multiple indices of physiological activity to access viability in chlorine disinfected Escherichia Coli O157: H7, Lett. Appl. Microbiol., 1999, 29(1), 42–47.
M. K. Ramseier, U. von Gunten, P. Freihofer and F. Hammes, Kinetics of membrane damage to high (HNA) and low (LNA) nucleic acid bacterial clusters in drinking water by ozone, chlorine, chlorine dioxide, monochloramine, Ferrate(VI), and Permanganate, Water Res., 2011, 45(3), 1490–1500.
A. K. Chatterjee, R. Chakraborty and T. Basu, Mechanism of antibacterial activity of copper nanoparticles, Nanotechnology, 2014, 25(13), 135101.
B. Yousuf, J. J. Ahire and L. M. Dicks, Understanding the antimicrobial activity behind thin-and thick-rolled copper plates, Appl. Microbiol. Biotechnol., 2016, 100(12), 5569–5580.
P. Wardman, Reduction potentials of one-electron couples involving free radicals in aqueous solution, J. Phys. Chem. Ref. Data, 1989, 18(4), 1637–1755.
G. V. Buxton, C. L. Greenstock, W. P. Helman and A. B. Ross, Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O− in aqueous solution, J. Phys. Chem. Ref. Data, 1988, 17(2), 513–886.
H. Semchyshyn, T. Bagnyukova, K. Storey and V. Lushchak, Hydrogen peroxide increases the activities of soxRS regulon enzymes and the levels of oxidized proteins and lipids in Escherichia coli, Cell Biol. Int., 2005, 29(11), 898–902.
A. G. Rincon and C. Pulgarin, Effect of pH, inorganic ions, organic matter and H2O2 on E. coli K12 photocatalytic inactivation by TiO2: implications in solar water disinfection, Appl. Catal., B, 2004, 51(4), 283–302.
H. E. Kim, T. T. Nguyen, H. Lee and C. Lee, Enhanced inactivation of Escherichia coli and MS2 coliphage by cupric ion in the presence of hydroxylamine: dual microbicidal effects, Environ. Sci. Technol., 2015, 49(24), 14416–14423.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available: FESEM, EDAX, XRD, UV-DRS, XPS, inactivation activity, HCHO production graph, enzymatic activity, dye degradation. See DOI: 10.1039/c8pp00460a
Equal contribution.
Rights and permissions
About this article
Cite this article
Dhandole, L.K., Seo, YS., Kim, SG. et al. A mechanism study on the photocatalytic inactivation of Salmonella typhimurium bacteria by CuxO loaded rhodium–antimony co-doped TiO2 nanorods. Photochem Photobiol Sci 18, 1092–1100 (2019). https://doi.org/10.1039/c8pp00460a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c8pp00460a