Abstract
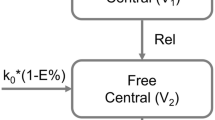
A second-generation chlorin-based photosensitizer, 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH) has shown tremendous therapeutic potential in clinical trials in the treatment of esophageal cancer. Herein, we have developed and validated a bioanalytical method for estimation of HPPH in rat plasma using High Performance Liquid Chromatography (HPLC) with a photo diode array (PDA) detector. The method was applied for carrying out pharmacokinetic study of HPPH. Further pharmacokinetic modeling was carried out to understand the compartment kinetics of HPPH. The developed method was fully validated as per the United States Food and Drug Administration (US-FDA) guidelines for bioanalytical method validation. The linearity of the method was in the range of 250–8000 ng mL−1, and the plasma recovery was found to be 70%. Pharmacokinetic parameters were evaluated and compared via non-compartment analysis and compartment modeling after the intravenous (i.v.) bolus administration in rats using Phoenix WinNonlin 8.0 (Certara™, USA). From the obtained results, we hypothesize that the HPPH complies with two compartmental pharmacokinetic model. Furthermore, it was observed that HPPH has the rapid distribution from the central compartment to peripheral compartment along with slow elimination from peripheral compartment.
Similar content being viewed by others
References
D. W. Felsher, Cancer revoked: oncogenes as therapeutic targets, Nat. Rev. Cancer, 2003, 3, 375.
D. E. Dolmans, D. Fukumura and R. K. Jain, Photodynamic therapy for cancer, Nat. Rev. Cancer, 2003, 3, 380.
M. A. M. Capella and L. S. Capella, A light in multidrug resistance: photodynamic treatment of multidrug-resistant tumors, J. Biomed. Sci., 2003, 10, 361–366.
T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, Photodynamic therapy, JNCI, J. Natl. Cancer Inst., 1998, 90, 889–905.
A. B. Ormond and H. S. Freeman, Dye sensitizers for photodynamic therapy, Materials, 2013, 6, 817–840.
R. K. Pandey, A. B. Sumlin, S. Constantine, M. Aoudia, W. R. Potter, D. A. Bellnier, B. W. Henderson, M. A. Rodgers, K. M. Smith and T. J. Dougherty, Alkyl ether analogs of chlorophyll-a derivatives: Part 1. Synthesis, photophysical properties and photodynamic efficacy, Photochem. Photobiol., 1996, 64, 194–204.
R. Shibata, T. Mizoguchi, T. Inazu and H. Tamiaki, Self-aggregation of synthetic zinc chlorophyll derivatives possessing multi-perfluoroalkyl chains in perfluorinated solvents, Photochem. Photobiol. Sci., 2007, 6, 749–757.
D. A. Bellnier, B. W. Henderson, R. K. Pandey, W. R. Potter and T. J. Dougherty, Murine pharmacokinetics and antitumor efficacy of the photodynamic sensitizer 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a, J. Photochem. Photobiol., B, 1993, 20, 55–61.
D. A. Bellnier, W. R. Greco, J. C. Parsons, A. R. Oseroff, A. Kuebler and T. J. Dougherty, An assay for the quantitation of Photofrin in tissues and fluids, Photochem. Photobiol., 1997, 66, 237–244.
E. Sevick-Muraca, M. Gurfinkel, A. B. Thompson, T. L. Troy, J. S. Reynolds, R. Mayer, D. J. Hawrysz, W. Ralston, B. Muggenberger and K. Nikula, others, Pharmacokinetics of ICG and HPPH-car for detection of normal and tumor tissue using fluorescence, near-infrared continuous wave imaging, in Biomed. Opt. Spectrosc. Diagnostics, 2000, p. MD7.
L. Chen, Q. Xiao, X. Zhang and J. Yang, Establishment and comparison of three novel methods for the determination of the photodynamic therapy agent 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH) in human serum, J. Pharm. Biomed. Anal., 2016, 121, 13–21.
C. Su, Y. Liu, Y. He and J. Gu, Analytical methods for investigating in vivo fate of nanoliposomes: A review, J. Pharm. Anal., 2018, 219–225.
K.V Krishna, R.N Saha, G. Singhvi and S.K Dubey, Preclinical pharmacokinetic-pharmacodynamic modelling and biodistribution studies of donepezil hydrochloride by a validated HPLC method, RSC Adv., 2018, 8, 24740–24749.
J. Lobel, I. J. MacDonald, M. J. Ciesielski, T. Barone, W. R. Potter, J. Pollina, R. J. Plunkett, R. A. Fenstermaker and T. J. Dougherty, 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH) in a nude rat glioma model: Implications for photodynamic therapy, Lasers Surg. Med., 2001, 29, 397–405.
I. da Costa César, F. H. A. Nogueira and G. A. Pianetti, Simultaneous determination of artemether and lumefantrine in fixed dose combination tablets by HPLC with UV detection, J. Pharm. Biomed. Anal., 2008, 48, 951–954.
N. Lindegårdh, A. Annerberg, D. Blessborn, Y. Bergqvist, N. Day and N. J. White, Development and validation of a bioanalytical method using automated solid-phase extraction and LC-UV for the simultaneous determination of lumefantrine and its desbutyl metabolite in plasma, J. Pharm. Biomed. Anal., 2005, 37, 1081–1088.
S. M. Moosavi, K. Shekar, J. Fraser, M. T. Smith and S. Ghassabian, High-throughput assay for quantification of the plasma concentrations of thiopental using automated solid phase extraction (SPE) directly coupled to LC-MS/MS instrumentation, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2016, 1038, 80–87.
U.S.D. of Health, H. Services, others, Guidance for industry, bioanalytical method validation, http//www.fda.gov/cder/guidance/index.htm, 2001.
U.S. Food, D. Administration, others, Guidance for Industry: Analytical Procedures and Methods Validation for Drugs and Biologics, US Food Drug Adm., 2015.
S.W.G. for Forensic Toxicology, Scientific Working Group for Forensic Toxicology (SWGTOX) standard practices for method validation in forensic toxicology, J. Anal. Toxicol., 2013, 37, 452–474.
K. Yamaoka, T. Nakagawa and T. Uno, Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations, J. Pharmacokinet. Pharmacodyn., 1978, 6, 165–175.
J.-K. Ho, T.-I. Huo, L.-C. Lin and T.-H. Tsai, Pharmacokinetics of ractopamine and its organ distribution in rats, J. Agric. Food Chem., 2014, 62, 9273–9278.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available. See DOI: 10.1039/c8pp00339d
Rights and permissions
About this article
Cite this article
Krishna, K.V., Saha, R.N., Puri, A. et al. Pre-clinical compartmental pharmacokinetic modeling of 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH) as a photosensitizer in rat plasma by validated HPLC method. Photochem Photobiol Sci 18, 1056–1063 (2019). https://doi.org/10.1039/c8pp00339d
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c8pp00339d