Abstract
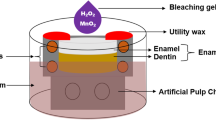
Dental bleaching is an important part of aesthetic dentistry. Various strategies have been created to enhance the bleaching efficacy. As one such strategy, light-activated nanoparticles that enable localized generation of reactive oxygen species have been developed. Here, we evaluated the cellular response to experimental gels containing these materials in in vitro models. L-929 cells, 3T3 cells, and gingival fibroblasts were exposed to the gels at 50%, 10%, 2%, 0.4%, 0.08%, 0.016%, and 0.0032%. The gels contained TiO2/Ag nanoparticles, TiO2 nanoparticles, hydrogen peroxide (6% hydrogen peroxide), or no added component and were tested with and without exposure to light. Cells were exposed to gels for 24 h or for 30 min. The latter case mimics the clinical situation of a short bleaching gel exposure. Metabolic activity and cell viability were evaluated with MTT and neutral red assays, respectively. We found a dose-dependent reduction of formazan formation and neutral red staining with gels containing TiO2/Ag nanoparticles or TiO2 nanoparticles in the 24 h setting with and without illumination. The strongest reduction, which was not dose-dependent in the evaluated concentrations, was found for the gel containing hydrogen peroxide. Gels with TiO2 nanoparticles showed a similar response to gel without particles. TiO2/Ag gel showed a slightly higher impact. When the gels were removed by rinsing after 30 min of exposure without light illumination, gel containing TiO2/Ag nanoparticles showed a stronger reduction of formazan formation and neutral red staining than gel containing TiO2 particles. Exposure of cells for 30 min under illumination and consequent rinsing off the gels also showed that Ag-containing particles can have a higher impact on the metabolic activity and viability than particles from TiO2. Overall our results show that experimental bleaching gels containing TiO2/Ag or TiO2 nanoparticles are less cytotoxic than hydrogen peroxide-containing gel. When gels are removed, gel containing TiO2/Ag particles exhibit a stronger reduction of metabolic activity and viability than the gel containing TiO2.
Similar content being viewed by others
Abbreviations
- Ag:
-
Silver
- CE:
-
European conformity
- GF:
-
Gingival fibroblasts
- h:
-
Hours
- H2O2:
-
Hydrogen peroxide
- illu. co.:
-
Illuminated control
- LED:
-
Light emitting diode
- mbar:
-
Millibar
- MD:
-
Maximal dose, defined as 50% (v/v)
- min:
-
Minutes
- NPs:
-
Nanoparticles
- SEM:
-
Scanning electron microscope
- TiO2:
-
Titanium dioxide
- UV:
-
Ultraviolet
- W/O:
-
Without (illumination)
References
C. D. Presoto, J. F. Bortolatto, P. P. F. de Carvalho, T. C. Trevisan, M. C. Floros and O. B. de Oliveira Junior, New Parameter for In-Office Dental Bleaching, Case Rep. Dent., 2016, 2016, 6034757.
A. Joiner and W. Luo, Tooth colour and whiteness: A review, J. Dent., 2017, 67S, S3–S10.
J. Montero, C. Gómez-Polo, J. A. Santos, M. Portillo, M. C. Lorenzo and A. Albaladejo, Contributions of dental colour to the physical attractiveness stereotype, J. Oral Rehabil., 2014, 41, 768–782.
A. Joiner, The bleaching of teeth: a review of the literature, J. Dent., 2006, 34, 412–419.
S. Gurgan, F. Y. Cakir and E. Yazici, Different light-activated in-office bleaching systems: a clinical evaluation, Lasers Med. Sci., 2010, 25, 817–822.
L. C. A. G. de Almeida, H. Riehl, P. H. dos Santos, M. L. M. M. Sundfeld and A. L. F. Briso, Clinical evaluation of the effectiveness of different bleaching therapies in vital teeth, Int. J. Periodontics Restorative Dent., 2012, 32, 303–309.
P. F. L. Dawson, M. O. Sharif, A. B. Smith and P. A. Brunton, A clinical study comparing the efficacy and sensitivity of home vs combined whitening, Oper. Dent., 2011, 36, 460–466.
C. R. Serraglio, L. Zanella, K. B. Dalla-Vecchia and S. A. Rodrigues Junior, Efficacy and safety of over-thecounter whitening strips as compared to home-whitening with 10% carbamide peroxide gel–systematic review of RCTs and metanalysis, Clin. Oral Investig., 2016, 20, 1–14.
R. J. G. De Moor, J. Verheyen, A. Diachuk, P. Verheyen, M. A. Meire, P. J. De Coster, F. Keulemans, M. De Bruyne and L. J. Walsh, Insight in the chemistry of laser-activated dental bleaching, Sci. World J., 2015, 2015, 650492.
J. Martín, P. Vildósola, C. Bersezio, A. Herrera, J. Bortolatto, J. R. C. Saad, O. B. Oliveira and E. Fernández, Effectiveness of 6% hydrogen peroxide concentration for tooth bleaching —A double-blind, randomized clinical trial, J. Dent., 2015, 43, 965–972.
B. M. Maran, A. Burey, T. de Paris Matos, A. D. Loguercio and A. Reis, In-office dental bleaching with light vs. without light: A systematic review and meta-analysis, J. Dent., 2018, 70, 1–13.
E. Klaric Sever, Z. Budimir, M. Cerovac, M. Stambuk, M. Par, D. Negovetic Vranic and Z. Tarle, Clinical and patient reported outcomes of bleaching effectiveness, Acta Odontol. Scand., 2018, 76, 30–38.
I. Luque-Martinez, A. Reis, M. Schroeder, M. A. Muñoz, A. D. Loguercio, D. Masterson and L. C. Maia, Comparison of efficacy of tray-delivered carbamide and hydrogen peroxide for at-home bleaching: a systematic review and meta-analysis, Clin. Oral Investig., 2016, 20, 1419–1433.
A. Majeed, I. Farooq, S. R. Grobler and M. H. Moola, In vitro evaluation of variances between real and declared concentration of hydrogen peroxide in various tooth-whitening products, Acta Odontol. Scand., 2015, 73, 387–390.
K. Chemin, M. Rezende, A. D. Loguercio, A. Reis and S. Kossatz, Effectiveness of and Dental Sensitivity to Athome Bleaching With 4 and 10 Hydrogen Peroxide: A Randomized, Triple-blind Clinical Trial, Oper. Dent., 2018, 43, 232–240.
M. Rezende, K. Chemin, S. C. Vaez, A. C. Peixoto, J. de F. Rabelo, S. S. L. Braga, A. L. Faria-E-Silva, G. R. da Silva, C. J. Soares, A. D. Loguercio and A. Reis, Effect of topical application of dipyrone on dental sensitivity reduction after in-office dental bleaching: A randomized, triple-blind multicenter clinical trial, J. Am. Dent. Assoc., 2018, 149, 363–371.
B. Rossi, P. M. Freitas, T. K. Tedesco, F. Gonçalves and L. S. Ferreira, Tooth color changes and sensitivity in patients undergoing dental bleaching with 10% hydrogen peroxide using customized trays or strips: a randomized clinical trial, Minerva Stomatol., 2018, 67, 55–61.
A. M. Kielbassa, M. Maier, A.-K. Gieren and E. Eliav, Tooth sensitivity during and after vital tooth bleaching: A systematic review on an unsolved problem, Quintessence Int., 2015, 46, 881–897.
C. J. Tredwin, S. Naik, N. J. Lewis and C. Scully, Hydrogen peroxide tooth-whitening (bleaching) products: review of adverse effects and safety issues, Br. Dent. J., 2006, 200, 371–376.
A. L. B. Jurema, M. Y. de Souza, C. R. G. Torres, A. B. Borges and T. M. F. Caneppele, Effect of pH on whitening efficacy of 35% hydrogen peroxide and enamel microhardness, J. Esthet. Restor. Dent., 2018, 30, E39–E44.
G. C. Lopes, L. Bonissoni, L. N. Baratieri, L. C. C. Vieira and S. Monteiro, Effect of bleaching agents on the hardness and morphology of enamel, J. Esthet. Restor. Dent., 2002, 14, 24–30.
R. F. Lia Mondelli, T. R. C. Garrido Gabriel, F. A. Piola Rizzante, A. C. Magalhães, J. F. Soares Bombonatti and S. K. Ishikiriama, Do different bleaching protocols affect the enamel microhardness?, Eur. J. Dent., 2015, 9, 25–30.
S. Naik, C. J. Tredwin and C. Scully, Hydrogen peroxide tooth-whitening (bleaching): review of safety in relation to possible carcinogenesis, Oral Oncol., 2006, 42, 668–674.
L. J. Walsh, Safety issues relating to the use of hydrogen peroxide in dentistry, Aust. Dent. J., 2000, 45, 257–269; quiz 289.
COUNCIL DIRECTIVE 2011/84/EU of 20 September 2011, amending Directive 76/768/EEC, concerning cosmetic products, for the purpose of adapting Annex III thereto to technical progress, Official Journal of the European Union, L 283/36–38.
R. J. G. De Moor, J. Verheyen, P. Verheyen, A. Diachuk, M. A. Meire, P. J. De Coster, M. De Bruyne and F. Keulemans, Laser teeth bleaching: evaluation of eventual side effects on enamel and the pulp and the efficiency in vitro and in vivo, Sci. World J., 2015, 2015, 835405.
J. F. Bortolatto, T. C. Trevisan, P. S. I. Bernardi, E. Fernandez, L. N. Dovigo, A. D. Loguercio, O. Batista de Oliveira Junior and H. Pretel, A novel approach for in-office tooth bleaching with 6% H2O2/TiO_N and LED/laser system-a controlled, triple-blinded, randomized clinical trial, Lasers Med. Sci., 2016, 31, 437–444.
M. A. Pérez-Díaz, L. Boegli, G. James, C. Velasquillo, R. Sánchez-Sánchez, R.-E. Martínez-Martínez, G. A. Martínez-Castañón and F. Martinez-Gutierrez, Silver nanoparticles with antimicrobial activities against Streptococcus mutans and their cytotoxic effect, Mater. Sci. Eng., C, 2015, 55, 360–366.
F. Martinez-Gutierrez, L. Boegli, A. Agostinho, E. M. Sánchez, H. Bach, F. Ruiz and G. James, Anti-biofilm activity of silver nanoparticles against different microorganisms, Biofouling, 2013, 29, 651–660.
K. Markowska, A. M. Grudniak and K. I. Wolska, Silver nanoparticles as an alternative strategy against bacterial biofilms, Acta Biochim. Pol., 2013, 60, 523–530.
G. Quiram, F. Montagner, K. L. Palmer, M. C. Stefan, K. E. Washington and D. C. Rodrigues, Novel Chlorhexidine-Loaded Polymeric Nanoparticles for Root Canal Treatment, J. Funct. Biomater., 2018, 9, pii: E29.
C. Covarrubias, D. Trepiana and C. Corral, Synthesis of hybrid copper-chitosan nanoparticles with antibacterial activity against cariogenic Streptococcus mutans, Dent. Mater. J., 2018, 37, 379–384.
J. M. Martinez-Andrade, M. Avalos-Borja, A. R. Vilchis-Nestor, L. O. Sanchez-Vargas and E. Castro-Longoria, Dual function of EDTA with silver nanoparticles for root canal treatment-A novel modification, PLoS One, 2018, 13, e0190866.
M. Coto, G. Divitini, A. Dey, S. Krishnamurthy, N. Ullah, C. Ducati and R. V. Kumar, Tuning the properties of a black TiO 2 -Ag visible light photocatalyst produced by a rapid one-pot chemical reduction, Mater. Today Chem., 2017, 4, 142–149.
A. Kishi, M. Otsuki, A. Sadr, M. Ikeda and J. Tagami, Effect of light units on tooth bleaching with visible-light activating titanium dioxide photocatalyst, Dent. Mater. J., 2011, 30, 723–729.
Y. Suyama, M. Otsuki, S. Ogisu, R. Kishikawa, J. Tagami, M. Ikeda, H. Kurata and T. Cho, Effects of light sources and visible light-activated titanium dioxide photocatalyst on bleaching, Dent. Mater. J., 2009, 28, 693–699.
K. Sakai, J. Kato, H. Kurata, T. Nakazawa, G. Akashi, A. Kameyama and Y. Hirai, The amounts of hydroxyl radicals generated by titanium dioxide and 3.5% hydrogen peroxide under 405-nm diode laser irradiation, Laser Phys., 2007, 17, 1062–1066.
C. Kurzmann, K. Janjić, H. Shokoohi-Tabrizi, M. Edelmayer, M. Pensch, A. Moritz and H. Agis, Evaluation of Resins for Stereolithographic 3D-Printed Surgical Guides: The Response of L929 Cells and Human Gingival Fibroblasts, BioMed Res. Int., 2017, 2017, 4057612.
K. Janjić, C. Kurzmann, A. Moritz and H. Agis, Expression of circadian core clock genes in fibroblasts of human gingiva and periodontal ligament is modulated by L-Mimosine and hypoxia in monolayer and spheroid cultures, Arch. Oral Biol., 2017, 79, 95–99.
J. CONSIDERATION, OECD GUIDELINE FOR TESTING OF CHEMICALS.
D. G. Soares, A. P. D. Ribeiro, F. da Silveira Vargas, J. Hebling and C. A. de Souza Costa, Efficacy and cytotoxicity of a bleaching gel after short application times on dental enamel, Clin. Oral Investig., 2013, 17, 1901–1909.
D. A. Tipton, S. D. Braxton and M. K. Dabbous, Role of saliva and salivary components as modulators of bleaching agent toxicity to human gingival fibroblasts in vitro, J. Periodontol., 1995, 66, 766–774.
D. A. Tipton, S. D. Braxton and M. K. Dabbous, Effects of a bleaching agent on human gingival fibroblasts, J. Periodontol., 1995, 66, 7–13.
P. Vildósola, J. Bottner, F. Avalos, I. Godoy, J. Martín and E. Fernández, Teeth bleaching with low concentrations of hydrogen peroxide (6%) and catalyzed by LED blue (450±10 nm) and laser infrared (808±10 nm) light for inoffice treatment: Randomized clinical trial 1-year follow-up, J. Esthet. Restor. Dent., 2017, 29, 339–345.
X. He, E. Hartlieb, L. Rothmund, J. Waschke, X. Wu, K. L. Van Landuyt, S.Milz, B. Michalke, R.Hickel, F.-X. Reichl and C. Högg, Intracellular uptake and toxicity of three different Titanium particles, Dent. Mater., 2015, 31, 734–744.
M. Składanowski, P. Golinska, K. Rudnicka, H. Dahm and M. Rai, Evaluation of cytotoxicity, immune compatibility and antibacterial activity of biogenic silver nanoparticles, Med. Microbiol. Immunol., 2016, 205, 603–613.
B. Cvikl, S. C. Hess, R. J. Miron, H. Agis, D. Bosshardt, T. Attin, P. R. Schmidlin and A. Lussi, Response of human dental pulp cells to a silver-containing PLGA/TCP-nanofabric as a potential antibacterial regenerative pulpcapping material, BMC Oral Health, 2017, 17, 57.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurzmann, C., Verheyen, J., Coto, M. et al. In vitro evaluation of experimental light activated gels for tooth bleaching. Photochem Photobiol Sci 18, 1009–1019 (2019). https://doi.org/10.1039/c8pp00223a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c8pp00223a