Abstract
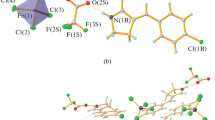
The synthesis and complexing properties of p-aminoazobenzene-derived mono-, bis-, and trisamides were described. Ligands 3 and 4 bind anions, including fluorides, chlorides, bromides, acetates, benzoates, dihydrogen phosphates, hydrogen sulfates, and p-toluenesulfonates, in chloroform forming 1: 1 complexes. The highest value of stability constant was evaluated for the 4-F− complex (log K = 5.63 ± 0.21). On the basis of 1H NMR, and FTIR spectroscopy, the possible nature of the ligand–anion interactions was proposed. The E ⇆ Z isomerization process of tripodal amide 4 in chloroform was studied. The effect of anions on Z to E thermal back isomerization was investigated.
Similar content being viewed by others
References
P. A. Gale, E. N. W. Howe and X. Wu, Anion receptor chemistry, Chemistry, 2016, 1, 351–422
S. K. Sahho, G.-D. Kim and H.-J. Choi, Optical sensing of anions using C3v-symmetric tripodal receptors, J. Photochem. Photobiol., C, 2016, 27, 30–53.
A. M. Wilson, P. J. Bailey, P. A. Tasker, J. R. Turkington, R. A. Grant and J. B. Love, Solvent Extraction: the coordi- nation chemistry behind extractive metallurgy, Chem. Soc. Rev., 2014, 43, 123–134
R. Alberto, G. Bergamaschi, H. Braband, T. Vox and V. Amendola, 99TcO4-: Selective recognition and trapping in aqueous solutions, Angew. Chem., Int. Ed., 2012, 51, 9772–9776.
N. Busschaert, C. Caltagirone, W. Van Rossom and P. A. Gale, Applications of supramolecular anion reco- gnition, Chem. Rev., 2015, 115, 8038–8155
D. Yuan, A. H. C. Anthis, M. G. Afshar, N. Pankratova, M. Cuartero, G. A. Crespo and E. Bakker, All-solid-state potentiometric sensors with a multiwalled carbon nanotube inner transdu- cing layer for anion detection in environmental samples, Anal. Chem., 2015, 87, 8640–8645.
I. T. Raheem, P. S. Thiara, E. A. Peterson and E. N. Jacobsen, Enantioselective Pictet-Spengler-type cycli- zations of hydroxylactams: H-bond donor catalysis of anion binding, J. Am. Chem. Soc., 2007, 129, 13404–13405
G. Bergonzini, C. S. Schindler, C.-J. Wallentin, E. N. Jacobsen and C. R. J. Stephenson, Chemical science photoredox activation and anion binding catalysis in the dual catalytic enantioselective synthesis of β-aminoesters, Chem. Sci., 2014, 5, 112–116.
H. Miyaji and J. L. Sessler, Off-the-shelf colorimetric anion sensors, Angew. Chem., Int. Ed., 2001, 40, 154–157
P. A. Gale and C. Caltagirone, Anion sensing by small molecules and molecular ensembles, Chem. Soc. Rev., 2015, 44, 4212–4227.
E. Merino, Synthesis of azobenzenes: the coloured pieces of molecular materials, Chem. Soc. Rev., 2011, 40, 3835–3853
E. Léonard, F. Mangin, C. Villette, M. Billamboz and C. Len, Azobenzenes and catalysis, Catal. Sci. Technol., 2016, 6, 379–398
F. Hamon, F. Djedaini-Pilard, F. Barbot and C. Len, Azobenzenes - synthesis and carbohydrate applications, Tetrahedron, 2009, 65, 10105–10123.
C. García-Iriepa, M. Marazzi, L. M. Frutos and D. Sampedro, E/Z Photochemical switches: syntheses, pro- perties and applications, RSC Adv., 2013, 3, 6241–6266
R. Ahmed, A. Priimagi, C. F. J. Faul and I. Manners, Redox-active, organometallic surface-relief gratings from azobenzene-containing polyferrocenylsilane block copoly- mers, Adv. Mater., 2012, 24, 926–931
A. A. Beharry, O. Sadovski and G. A. Woolley, Azobenzene photoswitching without ultraviolet light, J. Am. Chem. Soc., 2011, 133, 19684–19687
M. Dong, A. Babalhavaeji, S. Samanta, A. A. Beharry and G. A. Woolley, Red-shifting azobenzene photoswitches for in vivo use, Acc. Chem. Res., 2015, 48, 2662–2670
R. Reuter and H. A. Wegner, Oligoazobenzenophanes - synthesis, photochemistry and properties, Chem. Commun., 2011, 47, 12267–12276
I. Zawisza, R. Bilewicz, E. Luboch and J. F. Biernat, Complexation of metal ions by azocrown ethers in Langmuir-Blodgett monolayers, J. Chem. Soc., Dalton Trans., 2000, 4, 499–503.
E. Luboch, R. Bilewicz, M. Kowalczyk, E. Wagner-Wysiecka and J. F. Biernat, Azo Macrocyclic Compounds, in Advances in Supramolecular Chemistry, ed. G. W. Gokel, Cerberus Press, Inc., South Miami, 2003, vol. 9, pp. 73–163
Z. Li, J. Liang, W. Xue, G. Liu, S. H. Liu and J. Yin, Switchable azo-macrocycles: from molecules to functionali- zation, Supramol. Chem., 2014, 26, 54–65
E. Luboch, J. F. Biernat, E. Muszalska and R. Bilewicz, 13-Membered crown ethers with azo or azoxy unit in the macrocycle- synthesis, membrane electrodes, voltammetry and Langmuir monolayers, Supramol. Chem., 1995, 5, 201–210
E. Luboch, J. F. Biernat, Y. A. Simonov and A. A. Dvorkin, Synthesis and electrode properties of 16-membered azo- and azoxycrown ethers. Structure of tri- benzo-16-azocrown-6, Tetrahedron, 1998, 54, 4977–4990
E. Luboch, E. Wagner-Wysiecka and J. F. Biernat, Chromogenic azocrown ethers with peripheral alkyl, alkoxy, hydroxy or dimethylamino group, J. Supramol. Chem., 2002, 2, 279–291
E. Luboch, E. Wagner-Wysiecka, Z. Poleska-Muchlado and V. C. Kravtsov, Synthesis and properties of azobenzocrown ethers with n-electron donor, or n-electron donor and n-electron acceptor group(s) on benzene ring(s), Tetrahedron, 2005, 61, 10738–10747
E. Luboch, E. Wagner-Wysiecka and T. Rzymowski, 4-Hexylresorcinol-derived hydroxyazobenzo- crown ethers as chromoionophores, Tetrahedron, 2009, 65, 10671–10678
E. Wagner-Wysiecka, T. Rzymowski, M. Szarmach, M. S. Fonari and E. Luboch, Functionalized azobenzocrown ethers as sensor materialsthe synthesis and ion binding properties, Sens. Actuators., B, 2013, 177, 913–923
M. Szarmach, E. Wagner-Wysiecka and E. Luboch, Rearrangement of azoxybenzocrowns into chro- mophoric hydroxyazobenzocrowns and the use of hydroxy- azobenzocrowns for the synthesis of ionophoric biscrown compounds, Tetrahedron, 2013, 69, 10893–10905
E. Luboch, M. Szarmach, M. Jeszke and N. Lukasik, New bis(azobenzocrown)s with dodecylmethylmalonyl linkers as ionophores for sodium selective potentiometric sensors, J. Inclusion Phenom. Macrocyclic Chem., 2016, 86, 323–335
E. Wagner-Wysiecka, M. Szarmach, J. Chojnacki, N. Lukasik and E. Luboch, Cation sensing by diphenyl- azobenzocrowns, J. Photochem. Photobiol., A, 2017, 333, 220–232.
M. Banghart, K. Borges, E. Isacoff, D. Trauner and R. H. Kramer, Light-activated ion channels for remote control of neuronal firing, Nat. Neurosci., 2004, 7, 1381–1386.
W. C. Lin, C. M. Davenport, A. Mourot, D. Vytla, C. M. Smith, K. A. Medeiros, J. J. Chambers and R. H. Kramer, Engineering a light-regulated GABA(A) recep- tor for optical control of neural inhibition, ACS Chem. Biol., 2014, 9, 1414–1419.
A. M. Ali, M. W. Forbes and G. A. Woolley, Optimizing the photocontrol of bZIP coiled coils with azobenzene cross- linkers: role of the crosslinking site, ChemBioChem, 2015, 16, 1757–1763.
J. García-Amorós and D. Velasco, Recent advances towards azobenzene-based light-driven real-time infor- mation-transmitting materials, Beilstein J. Org. Chem., 2012, 8, 1003–1017
T. Avellini, H. Li, A. Coskun, G. Barin, A. Trabolsi, A. N. Basuray, S. K. Dey, A. Credi, S. Silvi, J. F. Stoddart and M. Venturi, Photoinduced memory effect in a redox controllable bistable mechanical molecular switch, Angew. Chem., Int. Ed., 2012, 51, 1611–1615
E. Merino and M. Ribagorda, Control over molecular motion using the cis-trans photoisomerization of the azo group, Beilstein J. Org. Chem., 2012, 8, 1071–1090
H. M. D. Bandara and S. C. Burdette, Photoisomerization in different classes of azobenzene, Chem. Soc. Rev., 2012, 41, 1809–1825.
E. Wagner-Wysiecka and N. Lukasik, Anion recognition by N,N’-diarylalkanediamides, Tetrahedron Lett., 2012, 53, 6029–6034
E. Wagner-Wysiecka and J. Chojnacki, Chromogenic amides of pyridine-2,6-dicarboxylic acid as anion receptors, Supramol. Chem., 2012, 24, 684–695
N. Lukasik, E. Wagner-Wysiecka, V. Hubscher-Bruder, S. Michel, M. Bochenska and B. Kaminska, Naphthyl- vs. anthrylpyridine-2,6-dicarboxamides in cation binding studies. Synthesis and spectroscopic properties, Supramol. Chem., 2016, 28, 673–685.
Y. Li, Y. Wang and J. Wang, Microwave-assisted synthesis of amides from various amines and benzoyl chloride under solvent-free conditions: a rapid and efficient method for selective protection of diverse amines, Russ. J. Org. Chem., 2008, 44, 358–361.
L. M. Goldenberg, L. Kulikovsky, O. Kulikovska, J. Tomczyk and J. Stumpe, Thin layers of low molecular azobenzene materials with effective light-induced mass transport, Langmuir, 2010, 26, 2214–2217.
M. Kyvala and I. Lukeš, Program package “OPIUM” available (free of charge) at http://www.natur.cuni.cz/~kyvala/opium.html.
M. Thompson, Program package “ArusLab” available (free of charge) at http://www.arguslab.com/arguslab.com/ArgusLab.html.
K. Iwato, Composition for forming low-dielectric-constant film, insulating film, and electronic device, US 2008/ 0161532Al, 2008.
S. Lee, S. Oh, J. Lee, Y. Malpani, Y.-S. Jung, B. Kang, J. Y. Lee, K. Ozasa, T. Isoshima, S. Y. Lee, M. Hara, D. Hashizume and J.-M. Kim, Stimulus-responsive azo- benzene supramolecules: fibres, gels, and hollow spheres, Langmuir, 2013, 29, 5869–5877.
P. D. Beer, P. A. Gale and G. Z. Chen, Mechanisms of electrochemical recognition of cations, anions and neutral guest species by redox-active receptor molecules, Coord. Chem. Rev., 1999, 185-186, 3–36
K. Kaur, S. K. Mittal, S. K. Ashok Kumar, A. Kumar and S. Kumar, Viologen sub- stituted anthrone derivatives for selective detection of cyanide ions using voltammetry, Anal. Methods, 2013, 5, 5565–5571.
J. Kind, L. Kaltschnee, M. Layendecker and C. M. Thiele, Distinction of trans-cis, photoisomers with comparable optical properties in multiple-state photochromic systems - examining a molecule with three azobenzenes via in situ irradiation NMR spectroscopy, Chem. Commun., 2016, 52, 12506–12509.
K. Dabrowa, P. Niedbala and J. Jurczak, Anion-tunable control of thermal ZE isomerization in basic azobenzene receptors, Chem. Commun., 2014, 50, 15748–15751.
S. Shinkai, T. Nakaji, T. Ogawa, K. Shigematsu and O. Manabe, Photoresponsive crown ethers. 2. Photocontrol of ion extraction and ion-transport by a bis(crown ether) with a butterfly-like motion, J. Am. Chem. Soc., 1981, 103, 111–115.
Acknowledgments
The authors kindly acknowledge support from sources for science GUT Grants No. 031841 and 032406. The authors also thank Prof. Elżbieta Luboch and Prof. Jan F. Biernat for fruitful discussion, student Marta Hewelt for her experimental contribution and Koleta Majewska, MSc for technical support in the manuscript preparation. The authors thank the anonymous reviewers for their careful review, which helped to improve the quality of the above manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Łukasik, N., Wagner-Wysiecka, E. Anion binding by p-aminoazobenzene-derived aromatic amides: spectroscopic and electrochemical studies. Photochem Photobiol Sci 16, 1570–1579 (2017). https://doi.org/10.1039/c7pp00245a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c7pp00245a