Abstract
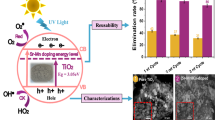
The photocatalytic degradation mechanism of Amaranth, a recalcitrant carcinogenic azo dye, was investigated using mesoporous anatase TiO2 under sunlight. Mesoporous anatase TiO2 of a high photocatalytic activity has been synthesized using a sol–gel method and its photocatalytic activity for the degradation of Amaranth dye has been evaluated with respect to Degussa P25. The effect of bi-dentate complexing agents like oxalic acid, ethylene glycol and urea on the surface properties of TiO2 catalyst has been investigated using TG-DTA, FTIR, HR-TEM, SAED, PXRD, EDS, UV-DRS, PL, BET N2 adsorption–desorption isotherm studies and BJH analysis. The influence of catalyst properties such as the mesoporous network, pore volume and surface area on the kinetics of degradation of Amaranth as a function of irradiation time under natural sunlight has been monitored using UV-Vis spectroscopy. The highest rate constant value of 0.069 min-1 was obtained for the photocatalytic degradation of Amaranth using TiO2 synthesized via a urea assisted sol–gel synthesis method. The effect of the reaction conditions such as pH, TiO2 concentration and Amaranth concentration on the photodegradation rate has been investigated. The enhanced photocatalytic activity of synthesized TiO2 in comparison with P25 is attributed to the mesoporous nature of the catalyst leading to increased pore diameter, pore volume, surface area and enhanced charge carrier separation efficiency. New intermediates of photocatalytic degradation of Amaranth, namely, sodium-3-hydroxynaphthalene-2,7-disulphonate, 3-hydroxynaphthalene, sodium-4-aminonaphthalenesulphonate and sodium-4-aminobenzenesulphonate have been identified using LC-ESI-MS for the very first time, providing direct evidence for simultaneous bond cleavage pathways (–C–N–) and (–N?N–). A new plausible mechanism of TiO2 catalysed photodegradation of Amaranth along with the comparison of its toxicity to that of its degradation intermediates and products is proposed.
Similar content being viewed by others
References
W. S. Pereira and R. S. Freire, Azo Dye Degradation by Recycled Waste Zero-Valent Iron Powder, J. Braz. Chem. Soc., 2006, 217(5), 832–838.
D. H. Mohsin, A. M. Juda and M. S. Mashkour, Thermodynamic and Kinetic Study for Aromatic Rings Effect on the Photooxidation rate, IJET-IJENS, 2013, 13, 34–41.
R. Ameta, N. Jain and S. Kothari, Photocatalytic bleaching of amaranth dye over ZnO nanopowder, Indian J. Chem. Technol., 2004, 11, 423–426.
N. Divya, A. Bansal and A. K. Jana, Photocatalytic activity of transition metal ion doped titania for Amaranth dye degradation, Mater. Sci. Forum, 2012, 712, 85–104.
E. M. Elgendy and N. A. Al-Zahrani, Comparative Study of Natural and Synthetic Food Additive Dye Amaranth through Photochemical Reactions, Indian J. Sci. Res., 2015, 4, 827–832.
H. Sudrajat, S. Babel, H. Sakai and S. Takizawa, Rapid photocatalytic degradation of the recalcitrant dye amaranth by highly active N-WO3, Environ. Chem. Lett., 2015, 165, 224–234.
H. P. Shivaraju, Removal of Organic Pollutants in the Municipal Sewage Water by TiO2 based Heterogeneous Photocatalysis, Indian J. Environ. Sci., 2011, 1, 911–923.
K. Sivaranjani and C. S. Gopinath, Porosity driven photocatalytic activity of wormhole mesoporous TiO2-xNx in direct sunlight, J. Mater. Chem., 2011, 21, 2639–2647.
P. Devaraji, N. K. Sathu and C. S. Gopinath, Ambient Oxidation of Benzene to Phenol by Photocatalysis on Au/Ti0.98 V0.02O2: Role of Holes, ACS Catal., 2014, 4, 2844–2853.
R. Liu, H. S. Wu, R. Yeh, C. Y. Lee and Y. Hung, Synthesis and Bactericidal Ability of TiO2 and Ag-TiO2 Prepared by Coprecipitation Method, Int. J. Photoenergy, 2012, 1–7.
K. Thangavelu, R. Annamalai and D. Arulnandhi, Preparation and Characterization of Nanosized TiO2 Powder by Sol-Gel Precipitation Route, IJETAE, 2013, 3, 636–639.
Y. Ali and A. Ameta, Degradation and decolouration of amaranth Dye by photo-fenton and fenton reagents: A comparative study, Int. J. Chem. Sci., 2013, 11(3), 1277–1285.
V. K. Gupta, R. Jain, A. Mittal, T. A. Saleh, A. Nayak, S. Agarwal and S. Sikarwar, Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions, Mater. Sci. Eng., 2012, 32, 12–17.
A. K. Jain, S. Sharma and R. Ameta, Enhanced photocatalytic activity of N, S-doped titania for degradation of Amaranth, Merit Res. J. Environ. Sci. Toxicol., 2015, 3, 25–30.
M. Karkmaz, E. Puzenat, C. Guillard and J. M. Herrmann, Photocatalytic degradation of the alimentary azo dye amaranth Mineralization of the azo group to nitrogen, Appl. Catal., B, 2004, 51, 183–194.
J. R. Steter, W. R. P. Barros, M. R. V. Lanza and A. J. Motheo, Degradation route for amaranth dye by sonoelectrochemical process using BDD anode, Chemosphere, 2014, 117, 200–207.
N. Hafizah and L. Sopyan, Nanosized TiO2 photocatalyst powder via sol-gel method: Effect of hydrolysis degree on powder properties, Int. J. Photoenergy, 2009, 1–8.
D. C. L. Vasconcelos, V. C. Costa, E. H. M. Nunes, A. C. S. Sabioni and W. L. Vasconcelos, Optical characterization of 316L stainless steel coated with sol–gel titania, J. Non-Cryst. Solids, 2012, 358, 3042–3047.
R. Sharmila Devi, R. Venckatesh and R. Sivaraj, Synthesis of Titanium Dioxide Nanoparticles by Sol-Gel Technique, Int. J. Innov. Res. Sci. Eng. Technol., 2014, 3(8), 15206–15211.
M. M. Ba-Abbad, A. A. H. Kadhum, A. B. Mohamad, M. S. Takriff and K. Sopian, Synthesis and catalytic activity of TiO2 nanoparticles for photochemical oxidation of concentrated chlorophenols under direct solar radiation, Int. J. Electrochem. Sci., 2012, 7, 4871–4888.
M. Sathish, B. Viswanathan, R. P. Viswanath and C. S. Gopinath, Synthesis Characterization Electronic Structure and Photocatalytic Activity of Nitrogen-Doped TiO2 nanocatalyst, Chem. Mater., 2005, 17, 6349–6353.
A. R. Ghande and J. B. Fernandes, A simple method to synthesize N-doped rutile titania with enhanced photocatalytic activity in sunlight, J. Solid State Chem., 2005, 178, 2953–2957.
N. Serpone, D. Lawless and R. Khairutdinov, Size Effects on the Photophysical Properties of Colloidal Anatase TiO2 Particles: Size Quantization versus Direct Transitions in This Indirect Semiconductor, J. Phys. Chem., 1995, 99(45), 16646–16654.
S. Mathew, A. K. Prasad, T. Benoy, P. P. Rakesh, H. Misha, T. M. Libish, P. Radhakrishnan, V. P. Nampoori and C. P. Vallabhan, UV-Visible Photoluminescence of TiO2 Nanoparticles Prepared by Hydrothermal Method, J. Fluoresc., 2012, 22(6), 1563–1569.
B. Choudhury, M. Dey and A. Choudhury, Shallow and deep trap emission and luminescence quenching of TiO2 nanoparticles on Cu doping, Appl. Nanosci., 2014, 4, 499–506.
Y. Lei, L. Zhang, G. Meng, G. Li, X. Zhang, C. Liang, W. Chen and S. Wang, Preparation and photoluminescence of highly ordered nanowire arrays, Appl. Phys. Lett., 2001, 78, 1125–1127.
Q. Wang, Z. Zhang, S. M. Zakeeruddin and M. Gratzel, Enhancement of the Performance of Dye-Sensitized Solar Cell by Formation of Shallow Transport Levels under Visible Light Illumination, J. Phys. Chem. C, 2008, 112(17), 7084–7092.
D. Behera, B. Bag and R. Sakthivel, Synthesis characterization and photoluminescence study of modified titania, Int. J. Pure Appl. Phys., 2011, 49, 754–758.
T. C. Jagadale, S. P. Takale, R. S. Sonawane, H. M. Joshi, S. I. Patil, B. B. Kale and S. B. Ogale, N-Doped TiO2 nanoparticle based visible light photocatalyst by modified peroxide sol-gel method, J. Phys. Chem. C, 2008, 112, 14595–14602.
K. Thamaphat, P. Limsuwan and B. Ngotaworncha, Kasetsart Phase characterization of TiO2 powder by XRD and TEM, J. Nat. Sci., 2008, 42, 357–361.
S. Dai, Y. Wu, T. Sakai, Z. Du, H. Sakai and M. Abe, Preparation of highly crystalline TiO2 nanostructures by acid-assisted hydrothermal treatment of hexagonal structured nanocrystalline Titania/Cetyltrimethyammonium Bromide nanoskeleton, Nanoscale Res. Lett., 2010, 5, 1829–1835.
M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. R. Reinoso, J. Rouquerol and K. S. W. Sing, Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report), Pure Appl. Chem., 2015, 1–19.
K. Vinodgopal, D. E. Wynkoop and P. V. Kamat, Environmental photochemistry on semiconductor surfaces: photosensitized degradation of a textile azo Dye, Acid orange 7, on TiO2 Particles Using Visible Light, Environ. Sci. Technol., 1996, 30(5), 1660–1666.
L. G. Devi, K. E. Rajashekhar, K. S. A. Raju and S. G. Kumar, Influence of various aromatic derivatives on the advanced photo Fenton degradation of Amaranth dye, Desalination, 2011, 270, 31–39.
M. M. Hashem, A. H. Atta, M. S. Arbid, S. A. Nada, S. M. Mouneir and G. F. Asaad, Toxicological impact of amaranth, sunset yellow and curcumin as food coloring agents in albino rats, J. Pioneering Med. Sci., 2011, 1(2), 43–51.
U.S. EPA, Health and Environmental Effects Profile for 1-Amino-2-Naphthol and 1-Amino-2-Naphthol Hydrochloride, U.S. Environmental Protection Agency, Washington, D.C., EPA/600/X-87/029 (NTIS PB89120315), 1986.
F. E. Field, G. Roberts, R. C. Hallowes, A. K. Palmer, K. E. Williams and J. B. Lloyd, Trypan blue: identification and teratogenic and oncogenic activities of its coloured constituents, Chem.-Biol. Interact., 1977, 16, 69–88.
R. D. Voyksner, R. Straub and J. T. Keever, Determination of aromatic amines originating from Azo Dyes by chemical reductlon Combined with liquid Chromatography/Mass Spectrometry, Environ. Sci. Technol., 1993, 27, 1665–1672.
H. Xu, T. M. Heinze, S. Chen, C. E. Cerniglia and H. Chen, Anaerobic metabolism of 1-Amino-2-Naphthol-Based Azo Dyes (Sudan Dyes) by human intestinal Microflora, Appl. Environ. Microbiol., 2007, 73, 7759–7762.
J. H. Weisburger, Mutagenic, Carcinogenic, and Chemopreventive Effects of Phenols and Catechols, ACS Symp. Ser., 1992, 507, 35–47.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available. See DOI: 10.1039/c7pp00090a
Rights and permissions
About this article
Cite this article
Naik, A.P., Salkar, A.V., Majik, M.S. et al. Enhanced photocatalytic degradation of Amaranth dye on mesoporous anatase TiO2: evidence of C–N, N=N bond cleavage and identification of new intermediates. Photochem Photobiol Sci 16, 1126–1138 (2017). https://doi.org/10.1039/c7pp00090a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c7pp00090a