Abstract
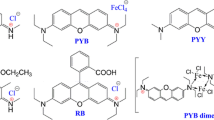
Intrinsic optical properties of several rhodamine cations were probed by measuring their dispersed fluorescence spectra in vacuo. Three different rhodamine structures were investigated, each with four different chalcogen heteroatoms. Fluorescence band maxima were blue-shifted by between 0.15 and 0.20 eV (1200-1600 cm4) relative to previous solution-phase measurements. Trends in emission wavelengths and fluorescence quantum yields previously measured in solution are generally reproduced in the gas phase, confirming the intrinsic nature of these effects. One important exception is gas-phase brightness of the Texas Red analogues, which is significantly higher than the other rhodamine structures studied, despite having similar fluorescence quantum yields in solution. These results expand the library of fluorophores for which gas-phase photophysical data is available, and will aid in the design of experiments utilizing gas-phase structural biology methods such as Forster resonance energy transfer.
Similar content being viewed by others
References
V. Frankevich, X. Guan, M. Dashtiev and R. Zenobi, Laser-induced fluorescence of trapped gas-phase molecular ions generated by internal-source matrix-assisted laser desorp-tion/ionization in a Fourier transform ion cyclotron resonance mass spectrometer, Eur. J. Mass Spectrom., 2005, 11, 475–482.
M. Kordel, D. Schooss, C. Neiss, L. Walter and M. M. Kappes, Laser-induced fluorescence of Rhodamine 6G. cations in the gas phase: a lower bound to the lifetime of the first triplet state, J. Phys. Chem. A, 2010, 114, 5509–5514.
M. W. Forbes and R. A. Jockusch, Gas-phase fluorescence excitation and emission spectroscopy of three xanthene dyes (rhodamine 575, rhodamine 590 and rhodamine 6G) in a quadrupole ion trap mass spectrometer, J. Am. Soc. Mass Spectrom., 2011, 22, 93–109.
R. Zenobi, Coming of Age: Gas-Phase Structural Information on Biomolecules by FRET, Anal. Chem., 2015, 87, 7497–7498.
A. S. Danell and J. H. Parks, FRET. measurements of trapped oligonucleotide duplexes, Int. J. Mass Spectrom., 2003, 229, 35–45.
M. Dashtiev, V. Azov, V. Frankevich, L. Scharfenberg and R. Zenobi, Clear evidence of fluorescence resonance energy transfer in gas-phase ions, J. Am. Soc. Mass Spectrom., 2005, 16, 1481–1487.
F. O. Talbot, A. Rullo, H. Yao and R. A. Jockusch, Fluorescence resonance energy transfer in gaseous, mass-selected polyproline peptides, J. Am. Chem. Soc, 2010, 132, 16156–16164.
S. Daly, F. Poussigue, A.-L. Simon, L. MacAleese, F. Bertorelle, F. Chirot, R. Antoine and P. Dugourd, Action-FRET: probing the molecular conformation of mass-selected gas-phase peptides with Forster resonance energy transfer detected by acceptor-specific fragmentation, Anal. Chem., 2014, 86, 8798–8804.
M. F. Czar, F. Zosel, I. Konig, D. Nettels, B. Wunderlich, B. Schuler, A. Zarrine-Afsar and R. A. Jockusch, Gas-phase FRET. efficiency measurements to probe the conformation of mass-selected proteins, Anal. Chem., 2015, 87, 7559–7565.
P. D. McQueen, S. Sagoo, H. Yao and R. A. Jockusch, On the intrinsic photophysics of fluorescein, Angew. Chem., Int. Ed., 2010, 49, 9193–9196.
H. Yao and R. A. Jockusch, Fluorescence and Electronic Action Spectroscopy of Mass-Selected Gas-Phase Fluorescein, 2, 7-DichIorofluorescein, and 2, 7-Difluorofluorescein Ions, J. Phys. Chem. A, 2013, 117, 1351–1359.
R. J. Nieckarz, J. Oomens, G. Berden, P. Sagulenko and R. Zenobi, Infrared multiple photon dissociation (IRMPD) spectroscopy of oxazine dyes, Phys. Chem. Chem. Phys., 2013, 15, 5049–5056.
S. V. Sciuto and R. A. Jockusch, The intrinsic photophysics of gaseous ethidium ions, J. Photochem. Photobiol, A, 2015, 311, 186–192.
M. W. Forbes and R. A. Jockusch, Deactivation pathways of an isolated green fluorescent protein model chromophore studied by electronic action spectroscopy, J. Am. Chem. Soc, 2009, 131, 17038–17039.
C. R. Mooney, D. A. Horke, A. S. Chatterley, A. Simperler, H. H. Fielding and J. R. Verlet, Taking the green fluorescence out of the protein: dynamics of the isolated GFP. chromophore anion, Chem. Sci., 2013, 4, 921–927.
M. Stockett, J. Houmøller and S. Brondsted Nielsen, Nile blue shows its true colors in gas-phase absorption and luminescence ion spectroscopy, J. Chem. Phys., 2016, 145, 104303.
M. H. Stockett, M. Boesen, J. Houmoller and S. Brondsted Nielsen, Communication: Accessing the intrinsic nature of electronic transitions from gas-phase spectroscopy of molecular ion - zwitterion complexes, Angew. Chem., Int. Ed., 2017, 56, 3490–3495.
Q. Bian, M. W. Forbes, F. O. Talbot and R. A. Jockusch, Gas-phase fluorescence excitation and emission spectroscopy of mass-selected trapped molecular ions, Phys. Chem. Chem. Phys., 2010, 12, 2590–2598.
J.-F. Greisch, M. E. Harding, M. Kordel, W. Klopper, M. M. Kappes and D. Schooss, Intrinsic fluorescence properties of rhodamine cations in gas-phase: triplet lifetimes and dispersed fluorescence spectra, Phys. Chem. Chem. Phys., 2013, 15, 8162–8170.
S. M. Wellman and R. A. Jockusch, Moving in on the action: an experimental comparison of fluorescence excitation and photodissociation action spectroscopy, J. Phys. Chem. A, 2015, 119, 6333–6338.
A. M. Nagy, F. O. Talbot, M. F. Czar and R. A. Jockusch, Fluorescence lifetimes of rhodamine dyes in vacuo, J. Photochem. Photobiol, A, 2012, 244, 47–53.
K. Chingin, R. M. Balabin, V. Frankevich, H. Chen, K. Barylyuk, R. Nieckarz, A. Fedorov and R. Zenobi, Optical properties of protonated Rhodamine 19 isomers in solution and in the gas phase, Phys. Chem. Chem. Phys., 2010, 12, 14121–14127.
J.-F. Greisch, M. E. Harding, W. Klopper, M. M. Kappes and D. Schooss, Effect of proton substitution by alkali ions on the fluorescence emission of Rhodamine B. cations in the gas phase, J. Phys. Chem. A, 2014, 118, 3787–3794.
R. P. Sabatini, M. F. Mark, D. J. Mark, M. W. Kryman, J. E. Hill, W. W. Brennessel, M. R. Detty, R. Eisenberg and D. W. McCamant, A comparative study of the photophysics of phenyl, thienyl, and chalcogen substituted rhodamine dyes, Photochem. Photobiol. Sci., 2016, 15, 1417–1432.
M. H. Stockett, J. Houmoller, K. Støchkel, A. Svendsen and S. Brøndsted Nielsen, A cylindrical quadrupole ion trap in combination with an electrospray ion source for gas-phase luminescence and absorption spectroscopy, Rev. Sci. Instrum., 2016, 87, 053103.
K. R. Mulhern, A. Orchard, D. F. Watson and M. R. Detty, Influence of surface-attachment functionality on the aggregation, persistence, and electron-transfer reactivity of chalcogenorhodamine dyes on TiO2, Langmuir, 2012, 28, 7071–7082.
T. Y. Ohulchanskyy, D. J. Donnelly, M. R. Detty and P. N. Prasad, Heteroatom substitution induced changes in excited-state photophysics and singlet oxygen generation in chalcogenoxanthylium dyes: Effect of sulfur and selenium substitutions, J. Phys. Chem. B, 2004, 108, 8668–8672.
S. J. Wagner, A. Skripchenko, D. J. Donnelly, K. Ramaswamy and M. R. Detty, Chalcogenoxanthylium photosensitizers for the photodynamic purging of blood-borne viral and bacterial pathogens, Bioorg. Med. Chem., 2005, 13, 5927–5935.
B. Calitree, D. J. Donnelly, J. J. Holt, M. K. Gannon, C. L. Nygren, D. K. Sukumaran, J. Autschbach and M. R. Detty, Tellurium analogues of rosamine and rhodamine dyes: synthesis, structure, 125Te NMR, and heteroatom contributions to excitation energies, Organometallics, 2007, 26, 6248–6257.
M. W. Kryman, G. A. Schamerhorn, J. E. Hill, B. D. Calitree, K. S. Davies, M. K. Linder, T. Y. Ohulchanskyy and M. R. Detty, Synthesis and properties of heavy chalcogen analogues of the Texas reds and related rhodamines, Organometallics, 2014, 33, 2628–2640.
J. R. Lakowitz, Principles of Fluorescence Spectroscopy, Kluwer Academic/Plenum Publishers, 1999.
V. Barone, J. Bloino, S. Monti, A. Pedone and G. Prampolini, Fluorescence spectra of organic dyes in solution: a time dependent multilevel approach, Phys. Chem. Chem. Phys., 2011, 13, 2160–2166.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stockett, M.H., Kjær, C., Linder, M.K. et al. Luminescence spectroscopy of chalcogen substituted rhodamine cations in vacuo. Photochem Photobiol Sci 16, 779–784 (2017). https://doi.org/10.1039/c7pp00049a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c7pp00049a