Abstract
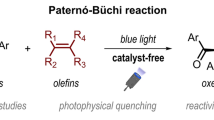
With the aim of developing photoreactions that use intermolecular hydrogen bonding interactions to control the efficiency and stereochemistry, the 1,2-[2 + 2] photocycloaddition reactions of 1-cyanonaphthalene derivatives with vinyl ethers possessing hydroxy groups were examined. The photoreaction of 1-cyano-4-(hydroxymethyl)naphthalene (1a) with 2-hydroxyethyl vinyl ether (2a) at room temperature was found to produce cycloadducts, endo-3aa and exo-3aa, in a non-stereoselective manner (56 : 44). However, this photoreaction at −40 °C displays a high endo-selectivity (81:19). In the reaction of 1a with ethyl vinyl ether (2b), high endo selectivity was observed both at room temperature and at −40 °C. The endo selectivity in the [2 + 2] photocycloaddition process is attributed to the intermolecular hydrogen bonding interactions between the reacting partners in the ground and excited states. Evidence to support this conclusion comes from the results of VT NMR and fluorescence quenching experiments, as well as the photoreactions of deuterated substrates.
Similar content being viewed by others
Notes and references
J. J. McCullough, Chem. Rev., 1987, 87, 811–860.
F. Müller and J. Mattay, Chem. Rev., 1993, 93, 99–117.
D. De Keukeleire and S.-L. He, Chem. Rev., 1993, 93, 359–380.
J. Cornelisse, Chem. Rev., 1993, 93, 615–669.
K. Mizuno, H. Maeda, A. Sugimoto and K. Chiyonobu, in Molecular and Supramolecular Photochemistry Vol. 8: Understanding and Manipulating Excited-State Processes, ed. V. Ramamurthy and K. S. Schanze, Marcel Dekker, Inc., New York, 2001, pp. 127–241.
H. Maeda and K. Mizuno, in CRC Handbook of Organic Photochemistry and Photobiology, ed. A. Griesbeck, M. Oelgemöller and F. Ghetti, CRC Press, Boca Raton, 3rd edn, 2012, vol. 1, pp. 489–509.
R. Remy and C. G. Bochet, Chem. Rev., 2016, 116, 9816–9849.
The Exciplex, ed. M. Gordon and W. R. Ware, Academic Press Inc., New York, 1975.
R. A. Caldwell and D. Creed, Acc. Chem. Res., 1980, 13, 45–50.
N. Mataga, Pure Appl. Chem., 1997, 69, 729–734.
V. Ramamurthy, Tetrahedron, 1986, 42, 5753–5839.
Y. Inoue, Chem. Rev., 1992, 92, 741–770.
L. K. Sydnes, K. I. Hansen, D. L. Oldroyd, A. C. Weedon and E. Jørgensen, Acta Chem. Scand., 1993, 47, 916–924.
C. Zhang and X.-C. Guo, Synth. Commun., 1994, 24, 3157–3165.
M. T. Crimmins and A. L. Choy, J. Am. Chem. Soc., 1997, 119, 10237–10238.
S. McN. Sieburth and K. F. McGee Jr., T. H. Al-Tel, J. Am. Chem. Soc., 1998, 120, 587–588.
T. Bach, H. Bergmann and K. Harms, J. Am. Chem. Soc., 1999, 121, 10650–10651.
W. Adam, K. Peters, E. M. Peters and V. R. Stegmann, J. Am. Chem. Soc., 2000, 122, 2958–2959.
T. Bach, H. Bergmann, J. Am. Chem. Soc., 2000, 122, 11525–11526.
M. D’Auria, L. Emanuele and R. Racioppi, Photochem. Photobiol. Sci., 2004, 3, 927–932.
K. Matsubayashi and Y. Kubo, J. Org. Chem., 2008, 73, 4915–4919.
M. Nagarathinam, A. M. P. Peedikakkal and J. J. Vittal, Chem. Commun., 2008, 5277–5288.
Y. Kawanami, T. C. S. Pace, J. Mizoguchi, T. Yanagi, M. Nishijima, T. Mori, T. Wada, C. Bohne and Y. Inoue, J. Org. Chem., 2009, 74, 7908–7921.
Y. Yabuno, Y. Hiraga, R. Takagi and M. Abe, J. Am. Chem. Soc., 2011, 133, 2592–2604.
A. Yokoyama and K. Mizuno, Org. Lett., 2000, 2, 3457–3459.
H. Maeda, K. Chiyonobu and K. Mizuno, Photochem. Photobiol. Sci., 2011, 10, 1445–1449.
C. Pac, T. Sugioka, K. Mizuno and H. Sakurai, Bull. Chem. Soc. Jpn., 1973, 46, 238–243.
1497074 contains the supplementary crystallographic data for endo-3ab.
W. A. Wisansky and S. Ansbacher, Org. Synth., 1948, 28, 46–48.
R. D. Topsom and J. Vaughan, J. Chem. Soc., 1957, 2842–2843.
L. Friedman and H. Shechter, J. Org. Chem., 1961, 26, 2522–2524.
T. Kunitake, S. Shinkai and C. Aso, Bull. Chem. Soc. Jpn., 1970, 43, 1109–1119.
C. G. Rao, Org. Prep. Proced. Int., 1980, 12, 225–228.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available: Variable-temperature 1H NMR spectra of a mixture of 1a–2b, 1a–2c, 1c–2a, and 1d–2a, fluorescence quenching experiments of 1a–b by 2a–c, and fluorescence decay of 1a–b. CCDC 1497074. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6pp00281a
Rights and permissions
About this article
Cite this article
Maeda, H., Takenaka, H. & Mizuno, K. Intermolecular hydrogen bonding controlled stereoselective photocycloaddition of vinyl ethers to 1-cyanonaphthalenes. Photochem Photobiol Sci 15, 1385–1392 (2016). https://doi.org/10.1039/c6pp00281a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c6pp00281a