Abstract
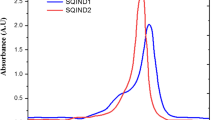
Two novel panchromatic asymmetrical squaraine sensitizers (SPSQ1 and SPSQ2) have been synthesized, characterized and effectively used for TiO2-based dye sensitized solar cells. In a solution, both dyes display a highly intense near-IR absorption (SPSQ1; 651 nm and SPSQ2; 692 nm), the red shifted absorption of SPSQ2 was attributed to the incorporation of the auxiliary acceptor dicyanovinyl unit on the squaraine moiety. Interestingly, the dicyanovinyl unit lowered the LUMO level of SPSQ2, which decreased the band gap and red shifted the absorption when compared to SPSQ1. These dyes possess suitable HOMO and LUMO levels to work as efficient sensitizers in DSSCs. The experimental trends in their optical and electrochemical properties are well matched with the theoretical calculations modeled by TDDFT. The blue and green color of the devices showed their complementary absorption and harvest a greater number of photons from solar flux. Under standard global AM 1.5 G solar conditions, the DSSC based on SPSQ2 exhibited a high power conversion efficiency of 3.1% with a high short circuit current density (JSC) attributed to the broadening of the IPCE spectra in the UV-vis and near-IR regions when compared to SPSQ1 (2.5%).
Similar content being viewed by others
References
B. O’Regan, M. Grätzel, A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films, Nature, 1991, 353, 737–740.
A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo, H. Pettersson, Dye-Sensitized Solar Cells, Chem. Rev., 2010, 110, 6595–6663.
M. K. Nazeeruddin, P. Pechy, T. Renouard, S. M. Zakeeruddin, R. Humphry-Baker, P. Comte, P. Liska, L. Cevey, E. Costa, V. Shklover, L. Spiccia, G. B. Deacon, C. A. Bignozzi, M. Grätzel, Engineering of Efficient Panchromatic Sensitizers for Nanocrystalline TiO2-Based Solar Cells, J. Am. Chem. Soc., 2001, 123, 1613–31624.
F. Gao, Y. Wang, D. Shi, J. Zhang, M. Wang, X. Jing, R. Humphry-Baker, P. Wang, S. M. Zakeeruddin, M. Grätzel, Enhance the Optical Absorptivity of Nanocrystalline TiO2 Film with High Molar Extinction Coefficient Ruthenium Sensitizers for High Performance Dye-Sensitized Solar Cells, J. Am. Chem. Soc., 2008, 130, 10720–10728.
Q. Yu, Y. Wang, Z. Yi, N. Zu, J. Zhang, M. Zhang, P. Wang, High-Efficiency Dye-Sensitized Solar Cells: The Influence of Lithium Ions on Exciton Dissociation, Charge Recombination, and Surface States, ACS Nano, 2010, 4, 6032–6038.
A. Mishra, M. K. R. Fischer, P. Bäuerle,, Metal-Free Organic Dyes for Dye-Sensitized Solar Cells: From Structure: Property Relationships to Design Rules, Angew. Chem., Int. Ed., 2009, 48, 2474–2499.
Y. Wu, W. Zhu, Organic Sensitizers from D-p-A to D-A-p-A: Effect of the Internal Electron-Withdrawing Units on Molecular Absorption, Energy Levels and Photovoltaic Performances, Chem. Soc. Rev., 2013, 42, 2039–32058.
B.-G. Kim, K. Chung, J. Kim, Molecular Design Principle of All-organic Dyes for Dye-Sensitized Solar Cells, Chem. - Eur. J., 2013, 19, 5220–5230.
M. Grätzel, Recent Advances in Sensitized Mesoscopic Solar Cells, Acc. Chem. Res., 2009, 42, 1788–1798.
Y. Ooyama, Y. Harima, Molecular Designs and Syntheses of Organic Dyes for Dye-Sensitized Solar Cells, Eur. J. Org. Chem., 2009, 2903–2934.
L. Cai, H. N. Tsao, W. Zhang, L. Wang, Z. Xue, M. Grätzel, B. Liu, Organic Sensitizers with Bridged Triphenylamine Donor Units for Efficient Dye-Sensitized Solar Cells, Adv. Energy Mater., 2013, 3, 200–205.
A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin, M. Grätzel, Porphyrin-Sensitized Solar Cells with Cobalt (II/III)-Based Redox Electrolyte Exceed 12 Percent Efficiency, Science, 2011, 334, 629–634.
C.-L. Mai, T. Moehl, C.-H. Hsieh, J.-D. Decoppet, S. M. Zakeeruddin, M. Grätzel, C.-Y. Yeh, Porphyrin Sensitizers Bearing a Pyridine-Type Anchoring Group for Dye-Sensitized Solar Cells, ACS Appl. Mater. Interfaces, 2015, 7, 14975–14982.
D. P. Hagberg, T. Marinado, K. M. Karlsson, K. Nonomura, P. Qin, G. Boschloo, T. Brinck, A. Hagfeldt, L. Sun, Tuning the HOMO and LUMO Energy Levels of Organic Chromophores for Dye Sensitized Solar Cells, J. Org. Chem., 2007, 72, 9550–9556.
M. Liang, J. Chen, Arylamine Organic Dyes for Dye-Sensitized Solar Cells, Chem. Soc. Rev., 2013, 42, 3453–3488.
T. Horiuchi, H. Miura, K. Sumioka, S. Uchida, High Efficiency of Dye-Sensitized Solar Cells Based on Metal-Free Indoline Dyes, J. Am. Chem. Soc., 2004, 126, 12218–312219.
Y. Wu, X. Zhang, W. Li, Z.-S. Wang, H. Tian, W. Zhu, Hexylthiophene-Featured D-A-p-A Structural Indoline Chromophores for Coadsorbent-Free and Panchromatic Dye-Sensitized Solar Cells, Adv. Energy Mater., 2012, 2, 149–156.
M. Akhtaruzzaman, A. Islam, F. Yang, N. Asao, E. Kwon, S. P. Singh, L. Han, Y. Yamamoto, A novel metal-free panchromatic TiO2 sensitizer based on a phenylenevinylene-conjugated unit and an indoline derivative for highly efficient dye-sensitized solar cells, Chem. Commun., 2011, 47, 12400–12402.
K. Hara, Z.-S. Wang, T. Sato, A. Furube, R. Katoh, H. Sugihara, Y. Dan-oh, C. Kasada, A. Shinpo, S. Suga, Oligothiophene-Containing Coumarin Dyes for Efficient Dye-Sensitized Solar Cells, J. Phys. Chem. B, 2005, 109, 15476–315482.
K. Hara, T. Sato, R. Katoh, A. Furube, Y. Ohga, A. Shinpo, S. Suga, K. Sayama, H. Sugihara, H. Arakawa, Molecular Design of Coumarin Dyes for Efficient Dye-Sensitized Solar Cells, J. Phys. Chem. B, 2003, 107, 597–3606.
M.-E. Ragoussi, J.-J. Cid, J.-H. Yum, G. D. L. Torre, D. D. Censo, M. Grätzel, M. K. Nazeeruddin, T. Torres, Carboxyethynyl Anchoring Ligands: A Means to Improving the Efficiency of Phthalocyanine-Sensitized Solar Cells, Angew. Chem., Int. Ed., 2012, 51, 4375–4378.
M. Ince, J.-H. Yum, Y. Kim, S. Mathew, M. Grätzel, T. Torres, M. K. Nazeeruddin, Molecular Engineering of Phthalocyanine Sensitizers for Dye-Sensitized Solar Cells, J. Phys. Chem. C, 2014, 118, 17166–17170.
C. Qin, W.-Y. Wong, L. Han, Squaraine Dyes for Dye-Sensitized Solar Cells: Recent Advances and Future Challenges, Chem. - Asian J., 2013, 8, 1706–1719.
B. T. Geiger, S. Kuster, J.-H. Yum, S.-J. Moon, M. K. Nazeeruddin, M. Grätzel, F. Nüesch, Molecular Design of Unsymmetrical Squaraine Dyes for High Efficiency Conversion of Low Energy Photons into Electrons Using TiO2Nanocrystalline Films, Adv. Funct. Mater., 2009, 19, 2720–2727.
Y. Shi, R. B. M. Hill, J.-H. Yum, A. Dualeh, S. Barlow, M. Grätzel, S. R. Marder, M. K. Nazeeruddin, A High-Efficiency Panchromatic Squaraine Sensitizer for Dye-Sensitized Solar Cells, Angew. Chem., Int. Ed., 2011, 50, 6619–6621.
A. Burke, L. Schmidt-Mende, S. Ito, M. Grätzel, A Novel Blue Dye for Near-IR ‘Dye-Sensitised’ Solar Cell Applications, Chem. Commun., 2007, 234–236.
L. Beverina, P. Salice, Squaraine Compounds: Tailored Design and Synthesis towards a Variety of Material Science Applications, Eur. J. Org. Chem., 2010, 1207–1225.
J. Park, C. Barolo, F. Sauvage, N. Barbero, C. Benzi, P. Quagliotto, S. Coluccia, D. D. Censo, M. Grätzel, M. K. Nazeeruddin, G. Viscardi, Symmetric vs. Asymmetric Squaraines as Photosensitisers in Mesoscopic Injection Solar Cells: a Structure-Property Relationship Study, Chem. Commun., 2012, 48, 2782–2784.
D. Yang, Q. Yang, L. Yang, Q. Luo, Y. Huang, Z. Lu, S. Zhao, Novel High Performance Asymmetrical Squaraines for Small Molecule Organic Solar Cells with a High Open Circuit Voltage of 1.12 V, Chem. Commun., 2013, 49, 10465–310467.
E. C. P. Smits, S. Setayesh, T. D. Anthopoulos, M. Buechel, W. Nijssen, R. Coehoorn, P. W. M. Blom, B. D. Boer, D. M. D. Leeuw, Near-Infrared Light-Emitting Ambipolar Organic Field-Effect Transistors, Adv. Mater., 2007, 19, 734–738.
Z. Xiang, E. E. Nesterov, J. Skoch, T. Lin, B. T. Hyman, T. M. Swager, B. J. Bacskai, S. A. Reeves, Detection of Myelination Using a Novel Histological Probe, J. Histochem. Cytochem., 2005, 53, 1511–1516.
J. Thomas, D. B. Sherman, T. J. Amiss, S. A. Andaluz, J. B. Pitner, Synthesis and Biosensor Performance of a Near-IR Thiol-Reactive Fluorophore Based on Benzothiazolium Squaraine, Bioconjugate Chem., 2007, 18, 1841–1846.
D. Ramaiah, A. Joy, N. Chandrasekhar, N. V. Eldho, S. Das, M. V. George, Halogenated Squaraine Dyes as Potential Photochemotherapeutic Agents. Synthesis and Study of Photophysical Properties and Quantum Efficiencies of Singlet Oxygen Generation, Photochem. Photobiol., 1997, 65, 783–790.
C.-T. Chen, S. R. Marder, L.-T. Cheng, Syntheses and Linear and Nonlinear Optical Properties of Unsymmetrical Squaraines with Extended Conjugation, J. Am. Chem. Soc., 1994, 116, 3117–3118.
C. Qin, Y. Numata, S. Zhang, A. Islam, X. Yang, K. Sodeyama, Y. Tateyama, L. Han, A Near-Infrared cis -Configured Squaraine Co-Sensitizer for High-Efficiency Dye-Sensitized Solar Cells, Adv. Funct. Mater., 2013, 23, 3782–3789.
A. Treibs, K. Jacob, Cyclotrimethine Dyes Derived from Squaric Acid, Angew. Chem., Int. Ed. Engl., 1965, 4, 694.
L. Beverina, R. Ruffo, C. M. Mari, G. A. Pagani, M. Sassi, F. D. Angelis, S. Fantacci, J.-H. Yum, M. Grätzel, M. K. Nazeeruddin, Panchromatic Cross-Substituted Squaraines for Dye-Sensitized Solar Cell Applications, ChemSusChem, 2009, 2, 621–3624.
T. Maeda, S. Mineta, H. Fujiwara, H. Nakao, S. Yagi, H. Nakazumi, Conformational Effect of Symmetrical Squaraine Dyes on the Performance of Dye-Sensitized Solar Cells, J. Mater. Chem. A, 2013, 1, 1303–1309.
S. Paek, H. Choi, C. Kim, N. Cho, S. So, K. Song, M. K. Nazeeruddin, J. Ko, Efficient and Stable Panchromatic Squaraine Dyes for Dye-Sensitized Solar Cells, Chem. Commun., 2011, 47, 2874–2876.
J.-Y. Li, C.-Y. Chen, C.-P. Lee, S.-C. Chen, T.-H. Lin, H.-H. Tsai, K.-C. Ho, C.-G. Wu, Unsymmetrical Squaraines Incorporating the Thiophene Unit for Panchromatic Dye-Sensitized Solar Cells, Org. Lett., 2010, 12, 5454–35457.
J.-Y. Li, C.-Y. Chen, W.-C. Ho, S.-H. Chen, C.-G. Wu, Unsymmetrical Squaraines Incorporating Quinoline for Near Infrared Responsive Dye-Sensitized Solar Cells, Org. Lett., 2012, 14, 5420–35423.
J. Park, N. Barbero, J. Yoon, E. Dell’Orto, S. Galliano, R. Borrelli, J.-H. Yum, D. D. Censo, M. Grätzel, M. K. Nazeeruddin, C. Barolo, G. Viscardi, Panchromatic Symmetrical Squaraines: A Step Forward in the Molecular Engineering of Low Cost Blue-Greenish Sensitizers for Dye-Sensitized Solar Cells, Phys. Chem. Chem. Phys., 2014, 16, 24173–324177.
F. M. Jradi, X. Kang, D. O’Neil, G. Pajares, Y. A. Getmanenko, P. Szymanski, T. C. Parker, M. A. El-Sayed, S. R. Marder, Near-Infrared Asymmetrical Squaraine Sensitizers for Highly Efficient Dye Sensitized Solar Cells: The Effect of p-Bridges and Anchoring Groups on Solar Cell Performance, Chem. Mater., 2015, 27, 2480–2487.
S. P. Singh, M. S. Roy, K. R. J. Thomas, S. Balaiah, K. Bhanuprakash, G. D. Sharma, New Triphenylamine-Based Organic Dyes with Different Numbers of Anchoring Groups for Dye-Sensitized Solar Cells, J. Phys. Chem. C, 2012, 116, 5941–5950.
S. P. Singh, K. S. V. Gupta, M. Chandrasekharam, A. Islam, L. Han, S. Yoshikawa, M.-A. Haga, M. S. Roy, G. D. Sharma, 2,6-Bis(1-methylbenzimidazol-2-yl)pyridine: A New Ancillary Ligand for Efficient Thiocyanate-Free Ruthenium Sensitizer in Dye-Sensitized Solar Cell Applications, ACS Appl. Mater. Interfaces, 2013, 5, 11623–11630.
P. Nagarjuna, K. Narayanaswamy, T. Swetha, G. H. Rao, S. P. Singh, G. D. Sharma, CH3NH3PbI3 Perovskite Sensitized Solar Cells Using a D-A Copolymer as Hole Transport Material, Electrochim. Acta, 2015, 151, 21–26.
B. Susrutha, L. Giribabu, S. P. Singh, Recent Advances in Flexible Perovskite Solar Cells, Chem. Commun., 2015, 51, 14696–314707.
T. Swetha, S. P. Singh, Perovskite Solar Cells Based on Small Molecule Hole Transporting Materials, J. Mater. Chem. A, 2015, 3, 18329–18344.
S. P. Singh, C. P. Kumar, G. D. Sharma, R. Kurchania, M. S. Roy, Synthesis of a Modified PC70BM and Its Application as an Electron Acceptor with Poly(3-hexylthiophene) as an Electron Donor for Efficient Bulk Heterojunction Solar Cells, Adv. Funct. Mater., 2012, 22, 4087–4095.
S. P. Singh, C. P. Kumar, P. Nagarjuna, G. D. Sharma, S. Biswas, J. A. Mikroyannidis, Diarylmethanofullerene: Efficient Polymer Solar Cells with Low-Band-Gap Copolymer, J. Phys. Chem. C, 2013, 117, 13350–13356.
L. Baumann, K. Schöller, D. D. Courten, D. Marti, M. Frenz, M. Wolf, R. M. Rossi, L. J. Scherer, Development of Light-Responsive Porous Polycarbonate Membranes for Controlled Caffeine Delivery, RSC Adv., 2013, 3, 23317–23326.
K. Sayama, S. Tsukagoshi, K. Hara, Y. Ohga, A. Shinpou, Y. Abe, S. Suga, H. Arakawa, Photoelectrochemical Properties of J Aggregates of Benzothiazole Merocyanine Dyes on a Nanostructured TiO2 Film, J. Phys. Chem. B, 2002, 106, 1363–1371.
D. P. Hagberg, T. Edvinsson, T. Marinado, G. Boschloo, A. Hagfeldt, L. Sun, A Novel Organic Chromophore for Dye-Sensitized Nanostructured Solar Cells, Chem. Commun., 2006, 2245–2247.
C. Teng, X. Yang, C. Yang, H. Tian, S. Li, X. Wang, A. Hagfeldt, L. Sun, Influence of Triple Bonds as p-Spacer Units in Metal-Free Organic Dyes for Dye-Sensitized Solar Cells, J. Phys. Chem. C, 2010, 114, 11305–11313.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford, CT, 2009.
A. D. A. Becke, A New Mixing of Hartree-Fock and Local Density Functional Theories, J. Chem. Phys., 1993, 98, 1372–1377.
J. Tomasi, B. Mennucci, R. Cammi, Quantum Mechanical Continuum Solvation Models, Chem. Rev., 2005, 105, 2999–3093.
M. Grätzel, Photoelectrochemical Cells, Nature, 2001, 414, 338–344.
C.-J. Yang, Y. J. Chang, M. Watanabe, Y.-S. Hon, T. J. Chow, Phenothiazine Derivatives as Organic Sensitizers for Highly Efficient Dye-Sensitized Solar Cells, J. Mater. Chem., 2012, 22, 4040–34049.
T. Maeda, N. Shima, T. Tsukamoto, S. Yagi, H. Nakazumi, Synthesis and Characterization of Triphenylamine-Based Organic Dyes for Dye-Sensitized Solar Cells, Synth. Met., 2011, 161, 2481–2487.
N. Koide, L. Han, Measuring Methods of Cell Performance of Dye-Sensitized Solar Cells, Rev. Sci. Instrum., 2004, 75, 2828–2831.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available. See DOI: 10.1039/c5pp00335k
Rights and permissions
About this article
Cite this article
Rao, G.H., Venkateswararao, A., Giribabu, L. et al. Near-infrared unsymmetrical blue and green squaraine sensitizers. Photochem Photobiol Sci 15, 287–296 (2016). https://doi.org/10.1039/c5pp00335k
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c5pp00335k