Abstract
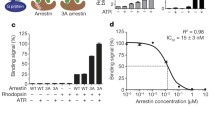
Light-induced helical rearrangement of vertebrate visual rhodopsin was directly monitored by high-angle X-ray scattering (HAXS), ranging from Q (= 4π?sin?θ/λ) = 0.03 Å−1 to Q = 1.5 Å−1. HAXS of nanodiscs containing a single rhodopsin molecule was performed before and after photoactivation of rhodopsin. The intensity difference curve obtained by HAXS agreed with that calculated from the crystal structure of dark state rhodopsin and metarhodopsin II, indicating that the conformational change of monomeric rhodopsin in the membrane is consistent with that occurring in the crystal. On the other hand, the HAXS intensity difference curve of nanodiscs containing two rhodopsin molecules was significantly reduced, similar to that calculated from the crystal structure of the deprotonated intermediate, without a large conformational change. These results suggest that rhodopsin is dimerized in the membrane and that the interaction between rhodopsin molecules modulates structural changes.
Similar content being viewed by others
References
K. Palczewski, T. Kumasaka, T. Hori, C. A. Behnke, H. Motoshima, B. A. Fox, I. Le Trong, D. C. Teller, T. Okada, R. E. Stenkamp, M. Yamamoto, M. Miyano, Crystal structure of rhodopsin: A G protein-coupled receptor, Science, 2000, 289, 739.
X. Deupi, J. Standfuss, Structural insights into agonist-induced activation of G-protein-coupled receptors, Curr. Opin. Struct. Biol., 2011, 21, 541.
J. H. Park, P. Scheerer, K. P. Hofmann, H. W. Choe, O. P. Ernst, Crystal structure of the ligand-free G-protein-coupled receptor opsin, Nature, 2008, 454, 183.
P. Scheerer, J. H. Park, P. W. Hildebrand, Y. J. Kim, N. Krauss, H. W. Choe, K. P. Hofmann, O. P. Ernst, Crystal structure of opsin in its G-protein-interacting conformation, Nature, 2008, 455, 497.
H. W. Choe, Y. J. Kim, J. H. Park, T. Morizumi, E. F. Pai, N. Krauss, K. P. Hofmann, P. Scheerer, O. P. Ernst, Crystal structure of metarhodopsin II, Nature, 2011, 471, 651.
X. Deupi, P. Edwards, A. Singhal, B. Nickle, D. Oprian, G. Schertler, J. Standfuss, Stabilized G protein binding site in the structure of constitutively active metarhodopsin-II, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 119.
J. Standfuss, P. C. Edwards, A. D’Antona, M. Fransen, G. Xie, D. D. Oprian, G. F. Schertler, The structural basis of agonist-induced activation in constitutively active rhodopsin, Nature, 2011, 471, 656.
D. Salom, D. T. Lodowski, R. E. Stenkamp, I. Le Trong, M. Golczak, B. Jastrzebska, T. Harris, J. A. Ballesteros, K. Palczewski, Crystal structure of a photoactivated deprotonated intermediate of rhodopsin, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 16123.
S. G. Rasmussen, B. T. DeVree, Y. Zou, A. C. Kruse, K. Y. Chung, T. S. Kobilka, F. S. Thian, P. S. Chae, E. Pardon, D. Calinski, J. M. Mathiesen, S. T. Shah, J. A. Lyons, M. Caffrey, S. H. Gellman, J. Steyaert, G. Skiniotis, W. I. Weis, R. K. Sunahara, B. K. Kobilka, Crystal structure of the β2 adrenergic receptor-Gs protein complex, Nature, 2011, 477, 549.
A. K. Shukla, A. Manglik, A. C. Kruse, K. Xiao, R. I. Reis, W. C. Tseng, D. P. Staus, D. Hilger, S. Uysal, L. Y. Huang, M. Paduch, P. Tripathi-Shukla, A. Koide, S. Koide, W. I. Weis, A. A. Kossiakoff, B. K. Kobilka, R. J. Lefkowitz, Structure of active β-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide, Nature, 2013, 497, 137.
R. Maeda, M. Hiroshima, T. Yamashita, A. Wada, S. Nishimura, Y. Sako, Y. Shichida, Y. Imamoto, Single-molecule observation of the ligand-induced population shift of rhodopsin, a G-protein-coupled receptor, Biophys. J., 2014, 106, 915.
R. Vogel, F. Siebert, Conformations of the active and inactive states of opsin, J. Biol. Chem., 2001, 276, 38487.
B. Knierim, K. P. Hofmann, O. P. Ernst, W. L. Hubbell, Sequence of late molecular events in the activation of rhodopsin, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 20290.
M. Mahalingam, K. Martinez-Mayorga, M. F. Brown, R. Vogel, Two protonation switches control rhodopsin activation in membranes, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 17795.
Y. Yamazaki, T. Nagata, A. Terakita, H. Kandori, Y. Shichida, Y. Imamoto, Mapping of the local environmental changes in proteins by cysteine scanning, Biophysics, 2014, 10, 1.
Y. Yamazaki, T. Nagata, A. Terakita, H. Kandori, Y. Shichida, Y. Imamoto, Intramolecular interactions that Induce helical rearrangement upon rhodopsin activation: Light-induced structural changes in metarhodopsin IIa probed by cysteine S-H stretching vibrations, J. Biol. Chem., 2014, 289, 13792.
E. Malmerberg, M. B.-G. PH, G. Katona, X. Deupi, D. Arnlund, C. Wickstrand, L. C. Johansson, S. Westenhoff, E. Nazarenko, X. S. GF, A. Menzel, W. J. de Grip, R. Neutze, Conformational activation of visual rhodopsin in native disc membranes, Sci. Signaling, 2015, 8, ra26.
M. Andersson, E. Malmerberg, S. Westenhoff, G. Katona, M. Cammarata, A. B. Wohri, L. C. Johansson, F. Ewald, M. Eklund, M. Wulff, J. Davidsson, R. Neutze, Structural dynamics of light-driven proton pumps, Structure, 2009, 17, 1265.
M. Kataoka, Y. Goto, X-ray solution scattering studies of protein folding, Folding Des., 1996, 1, R107.
T. H. Bayburt, Y. V. Grinkova, S. G. Sligar, Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins, Nano Lett., 2002, 2, 853.
K. Kojima, Y. Imamoto, R. Maeda, T. Yamashita, Y. Shichida, Rod visual pigment optimizes active state to achieve efficient G protein activation as compared with cone visual pigments, J. Biol. Chem., 2014, 289, 5061.
H. Tsukamoto, A. Sinha, M. DeWitt, D. L. Farrens, Monomeric rhodopsin is the minimal functional unit required for arrestin binding, J. Mol. Biol., 2010, 399, 501.
Y. Imamoto, I. Seki, T. Yamashita, Y. Shichida, Efficiencies of activation of transducin by cone and rod visual pigments, Biochemistry, 2013, 52, 3010–3018.
Y. Imamoto, Y. Shichida, Thermal recovery of iodopsin from photobleaching intermediates, Photochem. Photobiol., 2008, 84, 941.
F. J. Bartl, R. Vogel, Structural and functional properties of metarhodopsin III: recent spectroscopic studies on deactivation pathways of rhodopsin, Phys. Chem. Chem. Phys., 2007, 9, 1648.
B. König, W. Welte, K. P. Hofmann, Photoactivation of rhodopsin and interaction with transducin in detergent micelles. Effect of ‘doping’ with steroid molecules, FEBS Lett., 1989, 257, 163.
J. H. Parkes, P. A. Liebman, Temperature and pH dependence of the metarhodopsin I-metarhodopsin II kinetics and equilibria in bovine rod disk membrane suspensions, Biochemistry, 1984, 23, 5054.
T. H. Bayburt, A. J. Leitz, G. Xie, D. D. Oprian, S. G. Sligar, Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins, J. Biol. Chem., 2007, 282, 14875.
T. Kawaguchi, T. Hamanaka, Y. Kito, X-ray diffraction pattern from internal structure of bovine rhodopsin, J. Biochem., 1996, 119, 396.
D. I. Svergun, C. Baberato, M. H. J. Koch, CRYSOL-a program to evaluate X-ray solution scattering of biological macromolecules from atomic coordinates, J. Appl. Crystallogr., 1995, 28, 768.
S. Banerjee, T. Huber, T. P. Sakmar, Rapid incorporation of functional rhodopsin into nanoscale apolipoprotein bound bilayer (NABB) particles, J. Mol. Biol., 2008, 377, 1067.
J. J. Ruprecht, T. Mielke, R. Vogel, C. Villa, G. F. X. Schertler, Electron crystallography reveals the structure of metarhodopsin I, EMBO J., 2004, 23, 3609.
E. Zaitseva, M. F. Brown, R. Vogel, Sequential rearrangement of interhelical networks upon rhodopsin activation in membranes: the Meta IIa conformational substate, J. Am. Chem. Soc., 2010, 132, 4815.
H. Nakamichi, T. Okada, Crystallographic analysis of primary visual photochemistry, Angew. Chem., Int. Ed., 2006, 45, 4270.
H. Nakamichi, T. Okada, Local peptide movement in the photoreaction intermediate of rhodopsin, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12729.
B. Jastrzebska, T. Maeda, L. Zhu, D. Fotiadis, S. Filipek, A. Engel, R. E. Stenkamp, K. Palczewski, Functional characterization of rhodopsin monomers and dimers in detergents, J. Biol. Chem., 2004, 279, 54663.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Imamoto, Y., Kojima, K., Oka, T. et al. Helical rearrangement of photoactivated rhodopsin in monomeric and dimeric forms probed by high-angle X-ray scattering. Photochem Photobiol Sci 14, 1965–1973 (2015). https://doi.org/10.1039/c5pp00175g
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c5pp00175g