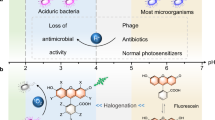
Abstract
Methylene blue (MB) and other photo-sensitizer molecules have been recognized as effective means for the inactivation of bacteria and other pathogens owing to their ability to photo-generate reactive oxygen species (ROS) including singlet oxygen. These reactive species react with the membrane of the bacteria causing their destruction. However, the efficiency of MB to destroy bacteria in plasma is very low because the MB 660 nm absorption band, that is responsible for the ROS generation, is bleached. The bleaching of MB, in plasma, is caused by the attachment of a hydrogen atom to the central ring nitrogen of MB, which destroys the ring conjugation and forms Leuco-MB which does not absorb in the 600 nm region. In this paper we show that addition of dilute acetic acid, ∼10−4 M, to human plasma, prevents H-atom attachment to MB, allowing MB to absorb at 660 nm, generates singlet oxygen and thus inactivates bacteria. The mechanism proposed, for preventing MB bleaching in plasma, is based on the oxidation of cysteine to cystine, by reaction with added dilute acetic acid, thus eliminating the availability of the thiol hydrogen atom which attaches to the MB nitrogen. It is expected that the addition of acetic acid to plasma will be effective in the sterilization of plasma and killing of bacteria in wounds and burns.
Similar content being viewed by others
References
R. J. Williams and D. L. Heymann, Containment of antibiotic resistance, Science, 1998, 279, 1153–1154.
T. T. Yoshikawa, Antimicrobial resistance and aging: Beginning of the end of the antibiotic era?, J. Am. Geriatr. Soc., 2002, 50, S226–S229.
R. E. W. Hancock, The end of an era?, Nat. Rev. Drug Discovery, 2007, 6, 28-28.
P. Nordmann, L. Poirel, M. A. Toleman and T. R. Walsh, Does broad-spectrum beta-lactam resistance due to NDM-1 herald the end of the antibiotic era for treatment of infections caused by Gram-negative bacteria?, J. Antimicrob. Chemother., 2011, 66, 689–692.
M. R. Hamblin and T. Hasan, Photodynamic therapy: a new antimicrobial approach to infectious disease?, Photochem. Photobiol. Sci., 2004, 3, 436–450.
M. Wainwright, Photodynamic antimicrobial chemotherapy (PACT), J. Antimicrob. Chemother., 1998, 42, 13–28.
B. Zeina, J. Greenman, W. M. Purcell and B. Das, Killing of cutaneous microbial species by photodynamic therapy, Br. J. Dermatol., 2001, 144, 274–278.
T. Dai, Y.-Y. Huang and M. R. Hamblin, Photodynamic therapy for localized infections—State of the art, Photodiagn. Photodyn. Ther., 2009, 6, 170–188.
J. Chen, T. C. Cesario and P. M. Rentzepis, Time resolved spectroscopic studies of methylene blue and phenothiazine derivatives used for bacteria inactivation, Chem. Phys. Lett., 2010, 498, 81–85.
F. Ronzani, A. Trivella, E. Arzoumanian, S. Blanc, M. Sarakha, C. Richard, E. Oliveros and S. Lacombe, Comparison of the photophysical properties of three phenothiazine derivatives: transient detection and singlet oxygen production, Photochem. Photobiol. Sci., 2013, 12, 2160–2169.
A. P. Castano, T. N. Demidova and M. R. Hamblin, Mechanisms in photodynamic therapy: part one—photosensitizers, photochemistry and cellular localization, Photodiagn. Photodyn. Ther., 2004, 1, 279–293.
J. Moan and K. Berg, The photodegradatio of porphyrins in cells can be used to estimate the lifetime of singlet oxygen, Photochem. Photobiol., 1991, 53, 549–553.
W. A. Pryor, Oxy-radicals and related species: their formation, lifetimes, and reactions, Annu. Rev. Physiol., 1986, 48, 657–667.
J. Chen, T. C. Cesario and P. M. Rentzepis, Effect of pH on methylene blue transient states and kinetics and bacteria photoinactivation, J. Phys. Chem. A, 2011, 115, 2702–2707.
P. Hellstern and B. G. Solheim, The Use of Solvent/Detergent Treatment in Pathogen Reduction of Plasma, Transfus. Med. Hemother., 2011, 38, 65–70.
J. P. R. Pelletier, S. Transue and E. L. Snyder, Pathogen inactivation techniques, Best Pract. Res., Clin. Haematol., 2006, 19, 205–242.
M. Wainwright, H. Mohr and W. H. Walker, Phenothiazinium derivatives for pathogen inactivation in blood products, J. Photochem. Photobiol., B, 2007, 86, 45–58.
A. O. Er, J. Chen, T. C. Cesario and P. M. Rentzepis, Inactivation of bacteria in plasma, Photochem. Photobiol. Sci., 2012, 11, 1700–1704.
S.-K. Lee and A. Mills, Novel photochemistry of leuco-methylene blue, Chem. Commun., 2003, 2366–2367.
S. J. Wagner, L. I. Friedman, R. Y. Dodd, Transfusion-associated bacterial sepsis, Clin. Microbiol. Rev., 1994, 7, 290–302.
M. T. G. Holden, E. J. Feil, J. A. Lindsay, S. J. Peacock, N. P. J. Day, M. C. Enright, T. J. Foster, C. E. Moore, L. Hurst, R. Atkin, A. Barron, N. Bason, S. D. Bentley, C. Chillingworth, T. Chillingworth, C. Churcher, L. Clark, C. Corton, A. Cronin, J. Doggett, L. Dowd, T. Feltwell, Z. Hance, B. Harris, H. Hauser, S. Holroyd, K. Jagels, K. D. James, N. Lennard, A. Line, R. Mayes, S. Moule, K. Mungall, D. Ormond, M. A. Quail, E. Rabbinowitsch, K. Rutherford, M. Sanders, S. Sharp, M. Simmonds, K. Stevens, S. Whitehead, B. G. Barrell, B. G. Spratt and J. Parkhill, Complete genomes of two clinical Staphylococcus aureus strains: Evidence for the rapid evolution of virulence and drug resistance, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 9786–9791.
K. E. Jones, N. G. Patel, M. A. Levy, A. Storeygard, D. Balk, J. L. Gittleman and P. Daszak, Global trends in emerging infectious diseases, Nature, 2008, 451, 990–993.
B. A. Lindig, M. A. J. Rodgers and A. P. Schaap, Determination of the lifetime of singlet oxygen in water-d2 using 9,10-anthracenedipropionic acid, a water-soluble probe, J. Am. Chem. Soc., 1980, 102, 5590–5593.
J. Chen, T. C. Cesario and P. M. Rentzepis, Rationale and mechanism for the low photoinactivation rate of bacteria in plasma, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 33–38.
P. W. Preisler, Kinetics of the reduction of cystine and related dithio (R-S-S-R) acids by reversible oxidation-reduction systems, J. Biol. Chem., 1930, 87, 767–784.
Y.-M. Go and D. P. Jones, Cysteine/cystine redox signaling in cardiovascular disease, Free Radicals Biol. Med., 2011, 50, 495–509.
M. P. Brigham, W. H. Stein and S. Moore, The concentration of cysteine and cystine in human blood plasma, J. Clin. Invest., 1960, 39, 1633–1638.
A. Pastore, R. Massoud, C. Motti, A. L. Russo, G. Fucci, C. Cortese and G. Federici, Fully automated assay for total homocysteine, cysteine, cysteinylglycine, glutathione, cysteamine, and 2-mercaptopropionylglycine in plasma and urine, Clin. Chem., 1998, 44, 825–832.
T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, Photodynamic Therapy, J. Natl. Cancer Inst., 1998, 90, 889–905.
T. Dai, G. P. Tegos, Z. Lu, L. Huang, T. Zhiyentayev, M. J. Franklin, D. G. Baer and M. R. Hamblin, Photodynamic therapy for Acinetobacter baumannii burn infections in mice, Antimicrob. Agents Chemother., 2009, 53, 3929–3934.
M. R. Hamblin, D. A. O’Donnell, N. Murthy, C. H. Contag and T. Hasan, Rapid control of wound infections by targeted photodynamic Therapy Monitored by In Vivo Bioluminescence Imaging, Photochem. Photobiol., 2002, 75, 51–57.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J., Cesario, T.C., Li, R. et al. The low photo-inactivation rate of bacteria in human plasma II. Inhibition of methylene blue bleaching in plasma and effective bacterial destruction by the addition of dilute acetic acid to human plasma. Photochem Photobiol Sci 14, 1880–1887 (2015). https://doi.org/10.1039/c5pp00042d
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c5pp00042d