Abstract
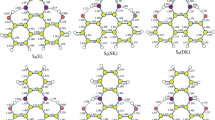
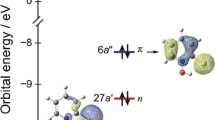
Minimum energy structures of the ground and lowest excited states of the phenol (PhOH)–pyridine (Py) hydrogen-bonded complex in the gas phase were determined by ab initio calculations. Photophysical and photochemical features of the complex under Cs symmetry (planar (Pl) and perpendicular (Pe) conformers) and without any symmetry constraints (unconstrained (Un) conformer) were studied with respect to nonradiative decay processes to the ground state. The mechanism involves internal conversion (IC) and intersystem crossing (ISC) along the O–H bond elongation coordinate, where a coupled electron/proton-transfer reaction plays a decisive role in the photophysics of this complex. For the Pl conformer, nonradiative decay proceeds from a locally excited 1pp*(LE) minimum over a conical intersection barrier (0.12 eV) to a charge-transfer (CT) minimum, which corresponds to a hydrogen-bonded PhO??HPy? biradical. Near this second minimum, a barrierless conical intersection 1A’(pp*(CT))–S0 funnels the electron population from the CT to the ground S0 state, completing the nonradiative deactivation. Calculations performed for the Pe and Un conformers confirmed that the same radiationless mechanism proceeds with no 1pp*(LE)/1pp*(CT) conical intersection near the Franck–Condon region. Furthermore, the population of the lowest triplet states via ISC and their contribution to the photophysics of PhOH–Py complex have been discussed. These findings appear to suggest that there is no single dominant path, but rather many distinct paths involving different quenching mechanisms.
Similar content being viewed by others
References
Hydrogen Bonding and Transfer in the Excited State, ed. K.-L. Han and G.-J. Zhao, Wiley, West Sussex, UK, 2011.
G. J. Zhao and K. L. Han, J. Phys. Chem. A, 2007, 111, 2469–2474.
G. J. Zhao and K. L. Han, Acc. Chem. Res., 2012, 45, 404.
A. L. Sobolewski and W. Domcke, Phys. Chem. Chem. Phys., 2004, 6, 2763–2771.
A. L. Sobolewski, W. Domcke and C. Hattig, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 17903–17906.
S. Yamazaki and T. Taketsugu, Phys. Chem. Chem. Phys., 2012, 14, 8866–8877.
S. Perun, A. L. Sobolewski and W. Domcke, J. Phys. Chem. A, 2006, 110, 9031–9038.
J. P. Gobbo, V. Saurí, D. Roca-Sanjuán, L. Serrano-Andrés, M. Merchán and A. C. Borin, J. Phys. Chem. B, 2012, 116, 4089–4097.
G. Groenhof, M. Boggio-Pasqua, M. Goette, H. Grubmüller and M. A. Robb, J. Am. Chem. Soc., 2007, 129, 6812–6819.
P. R. L. Markwick and N. L. Doltsinis, J. Chem. Phys., 2007, 126, 175102.
M. Barbatti, A. J. A. Aquino, J. J. Szymczak, D. Nachtigallová, P. Hobza and H. Lischka, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 21453.
C. E. Crespo-Hernandez, B. Cohen, J. Hare and B. Kohler, Chem. Rev., 2004, 104, 1977.
T. Förster, Elektrochem., 1950, 54, 531.
L. G. Arnaut and S. J. Formosinho, J. Photochem. Photobiol., A, 1993, 75, 1.
H. Tanaka and K. Nishimoto, J. Phys. Chem., 1984, 88, 1052.
N. Mataga, Pure Appl. Chem., 1984, 56, 1255.
N. Ideka, T. Okada and N. Mataga, Chem. Phys. Lett., 1980, 69, 251.
M. M. Martin, N. Ideka, T. Okada and N. Mataga, J. Phys. Chem., 1982, 86, 4148.
M. M. Martin, H. Miyasaka, A. Karen and N. Mataga, J. Phys. Chem., 1985, 89, 182–185.
N. Ideka, H. Miyasaka, T. Okada and N. Mataga, J. Am. Chem. Soc., 1983, 105, 5206–5211.
H. Miyasaka, A. Tabata, S. Ojima, N. Ideka and N. Mataga, J. Phys. Chem., 1993, 97, 8222.
N. Mataga and H. Miyasaka, Adv. Chem. Phys., 1999, 107, 431–496.
J. Waluk, Acc. Chem. Res., 2003, 36, 832.
A. L. Sobolewski and W. Domcke, J. Phys. Chem. A, 2007, 111, 11725.
M. F. Rode and A. L. Sobolewski, Chem. Phys., 2008, 347, 413.
L. M. Frutos, A. Markmann, A. L. Sobolewski and W. Domcke, J. Phys. Chem. B, 2007, 111, 6110.
Z. Lan, L. M. Frutos, A. L. Sobolewski and W. Domcke, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 12707–12712.
R. Ahlrichs, M. Bär, M. Häser, H. Horn, C. Kölmel, Chem. Phys. Lett., 1989, 162, 165.
F. Weigend, M. Haser, H. Patzelt and R. Ahlrichs, Chem. Phys. Lett., 1998, 294, 143.
C. Hätig, J. Chem. Phys., 2003, 118, 7751.
A. Köhn, C. Hätig, J. Chem. Phys., 2003, 119, 5021.
V. Poterya, L. Šištík, P. Slavícek, M. Fárník, Phys. Chem. Chem. Phys., 2012, 14, 8936.
Z. Lan, W. Domcke, V. Vallet, A. L. Sobolewski and S. Mahapatra, J. Chem. Phys., 2005, 122, 224315.
R. N. Dixon, T. A. A. Oliver and M. N. R. Ashfold, J. Chem. Phys., 2011, 134, 194303.
A. L. Sobolewski and W. Domcke, J. Phys. Chem. A, 2001, 105, 9275.
K. Daigoku, S. Ishiuchi, M. Sakai, M. Fujii and K. Hashimoto, J. Chem. Phys., 2003, 119, 5149.
M. Merchán, L. Serrano-Andrés, M. A. Robb, L. Blancafort L, J. Am. Chem. Soc., 2005, 127, 1820–1825.
T. Climent, R. González-Luque, M. Merchán, L. Serrano-Andrés, Chem. Phys. Lett., 2007, 441, 327–331.
J. J. Serrano-Pérez, R. González-Luque, M. Merchán, L. Serrano-Andrés, J. Phys. Chem. B, 2007, 111, 11880–11883.
G. A. Pino, A. N. Oldani, E. Marceca, M. Fujii, S. I. Ishiuchi, M. Miyazaki, M. Broquier, C. Dedonder and C. Jouvet, J. Chem. Phys., 2010, 133, 124313.
A. L. Sobolewski, W. Domcke, C. D. Lardeux and C. Jouvet, Phys. Chem. Chem. Phys., 2002, 4, 1093.
M. Chachivilis and A. H. Zewail, J. Phys. Chem. A, 1999, 103, 7408–7418.
Z. L. Cai and J. R. Reimers, J. Phys. Chem. A, 2000, 104, 8389–8408.
V. Hirata and N. Mataga, J. Phys. Chem., 1984, 88, 3091.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available. See DOI: 10.1039/c4pp00199k
Rights and permissions
About this article
Cite this article
Esboui, M., Jaidane, N. Non-radiative deactivation in phenol–pyridine complex: theoretical study. Photochem Photobiol Sci 14, 1127–1137 (2015). https://doi.org/10.1039/c4pp00199k
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c4pp00199k