Abstract
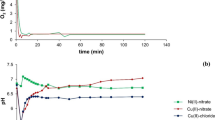
In this work, the photochemical degradation process of 17β-estradiol (E2) in a suspension of natural montmorillonite (NM) has been studied. The addition of the iron complexing agent ethylenediamine-N,N′-disuccinic acid (EDDS) to the suspension was investigated and compared with the system without EDDS. The effects of the main physicochemical parameters (pH, EDDS, oxygen and NM concentrations) on E2 degradation in NM suspensions were also investigated. In order to better understand the photochemical process, experiments were carried out in the presence of 2-propanol. In general, the photochemical efficiency of the E2 degradation is better in EDDS-NM suspensions than in NM suspensions, especially at higher pH. This work demonstrated that the NM-EDDS system is an interesting and valuable research area and could be considered as a promising photochemical system for wastewater treatment.
Similar content being viewed by others
References
G. Sposito, N. T. Skipper, R. Sutton, S.-h. Park, A. K. Soper and J. A. Greathouse, Surface geochemistry of the clay minerals, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 3358–3364.
J. M. Zen and A. S. Kumar, The prospects of clay mineral electrodes, Anal. Chem., 2004, 76, 205A–211A.
J. K. Thomas, Physical aspects of radiation-induced processes on SiO2, Al2O3, zeolites, and clays, Chem. Rev., 2005, 105, 1683–1734.
T. Shichi and K. Takagi, Clay minerals as photochemical reaction fields, J. Photochem. Photobiol., C, 2000, 1, 113–130.
T. Katagi, Photoinduced oxidation of the organophosphorus fungicide tolclofos-methyl on clay minerals, J. Agric. Food Chem., 1990, 38, 1595–1600.
T. Katagi, Photodegradation of esfenvalerate in clay suspensions, J. Agric. Food Chem., 1993, 41, 2178–2183.
J. Y. Feng, X. J. Hu and P. L. Yue, Discoloration and mineralization of Orange II by using a bentonite clay-based Fe nanocomposite film as a heterogeneous photo-Fenton catalyst, Water Res., 2005, 39, 89–96.
Q. Lan, F. B. Li, C. X. Sun, C. S. Liu and X. Z. Li, Heterogeneous photodegradation of pentachlorophenol and iron cycling with goethite, hematite and oxalate under UVA illumination, J. Hazard. Mater., 2010, 174, 64–70.
F. Gulshan, S. Yanagida, Y. Kameshima and T. Isobe, Various factors affecting photodecomposition of methylene blue by iron-oxides in an oxalate solution, Water Res., 2010, 44, 2876–2884.
J. Feng, X. Hu and P. L. Yue, Novel bentonite clay-based Fe-nanocomposite as a heterogeneous catalyst for Photo-Fenton discoloration and mineralization of orange II, Environ. Sci. Technol., 2004, 38, 269–275.
W. J. Song, M. M. Cheng, J. H. Ma, W. H. Ma, C. C. Chen and J. C. Zhao, Decomposition of hydrogen peroxide driven by photochemical cycling of iron species in clay, Environ. Sci. Technol., 2006, 40, 4782–4787.
M. M. Cheng, W. J. Song, W. H. Ma, C. C. Chen, J. C. Zhao, J. Lin and H. Y. Zhu, Catalytic activity of iron species in layered clays for photodegradation of organic dyes under visible irradiation, Appl. Catal., B: Environ., 2008, 77, 355–363.
F. Wu, J. Li, Z. E. Peng and N. S. Deng, Photochemical formation of hydroxyl radicals catalyzed by montmorillonite, Chemosphere, 2008, 72, 407–413.
J. Li, F. Wu, N. S. Deng, E. M. Glebov and N. M. Bazhin, Degradation of orange II by heterogeneous photocatalytic reaction using montmorillonite KSF, React. Kinet. Catal. Lett., 2008, 95, 247–255.
J. Li, F. Wu, G. Maihot and N. S. Deng, Photodegradation of chloroform in aqueous solution: impact of montmorillonite KSF particles, J. Hazard. Mater., 2010, 174, 368–374.
Y. X. Liu, J. Li, F. Wu, C. B. Zhang and N. S. Deng, Insight into heterogeneous photocatalytic degradation of phenol over montmorillonite KSF, Chem. Eng. Commun., 2008, 195, 1–10.
Y. X. Liu, X. Zhang, L. Guo, F. Wu and N. S. Deng, Photodegradation of Bisphenol A in the montmorillonite KSF suspended solutions, Ind. Eng. Chem. Res., 2008, 47, 7141–7146.
W. P. Gates, P. G. Slade, A. Manceau and B. Lanson, Site occupancies by iron in nontronites, Clays Clay Miner., 2002, 50, 223–239.
E. P. Achterberg, T. W. Holland, A. R. Bowie, R. F. C. Mantoura and P. J. Worsfold, Determination of iron in seawater, Anal. Chim. Acta, 2001, 442, 1–14.
P. Cieśla, P. Kocot, P. Mytych and Z. Stasicka, Homogeneous photocatalysis by transition metal complexes in the environment, J. Mol. Catal. A: Chem., 2004, 224, 17–33.
M. Bucheli-Witschel and T. Egli, Environmental fate and microbial degradation of aminopolycarboxylic acids, FEMS Microbiol. Rev., 2001, 25, 69–106.
N. J. Velupula, G. J. Tedros and M. C. Andrew, Determination of copper and iron using [S,S]-ethylenediaminedisuccinic acid as a chelating agent in wood pulp by capillary electrophoresis, Anal. Sci., 2007, 23, 493–496.
L. H. Zhang, Z. L. Zhu, R. H. Zhang, C. S. Zheng, H. Zhang, Y. L. Qiu and J. F. Zhao, Extraction of copper from sewage sludge using biodegradable chelant EDDS, J. Environ. Sci., 2008, 20, 970–974.
C. B. Zhang, Photodegradation of organic pollutants induced by iron-carboxylate complexes in aqueous solutions, Ph.D. thesis, Université Blaise Pascal, 2009.
J. Li, G. Maihot, F. Wu and N. S. Deng, Photochemical efficiency of Fe(iii)-EDDS complex: ˙OH radical production and 17β-estradiol degradation, J. Photochem. Photobiol., A, 2010, 212, 1–7.
S. F. Arnold, D. M. Klotz, B. M. Collins, P. M. Vonier Jr., L. J. Guillette and J. A. Mclachlan, Synergistic activation of estrogen receptor with combinations of environmental chemicals, Science, 1996, 272, 1489–1492.
Y. Ohko, K.-I. Iuchi, C. Niwa, T. Tatsuma, T. Nakashima, T. Iguchi, Y. Kubota and A. Fujishima, 17β-Estradiol degradation by TiO2 photocatalysis as a means of reducing estrogenic activity, Environ. Sci. Technol., 2002, 36, 4175–4181.
J. Lea and A. A. Adesina, The photo-oxidative degradation of sodium dodecyl sulphate in aerated aqueous TiO2 suspension, J. Photochem. Photobiol., A, 1998, 118, 111–122.
H.-J. Benkelberg and P. Warneck, Photodecomposition of iron(iii) hydroxo and sulfato complexes in aqueous solution: wavelength dependence of OH and SO4− quantum yields, J. Phys. Chem., 1995, 99, 5214–5221.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, J., Mailhot, G., Wu, F. et al. Photodegradation of E2 in the presence of natural montmorillonite and the iron complexing agent ethylenediamine-N,N′-disuccinic acid. Photochem Photobiol Sci 11, 1880–1885 (2012). https://doi.org/10.1039/c2pp25159k
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c2pp25159k