Abstract
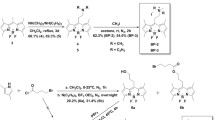
Fluorosolvatochromic probes have recently attracted interest for live cell imaging. We recently tested our quinoxaline-based fluorosolvatochromic probes, PQX and MPQX, for in vivo imaging applications.PQX and MPQX are characterized by donor (pyrrole site)–acceptor (quinoxaline site) structure, and the peak emission wavelength of these compounds varies over the visible wavelength range depending on the polarity of the solvent used, for a variety of solvents from hexane to water. A linear relationship was obtained between peak emission wavenumber and ETN (normalized solvent polarity). When tested on cultured HEp-2 cells, the results showed that thePQX and MPQX were not harmful at the applied concentrations (10-5 M), and site-dependent fluorescence spectra in living cells were observed withPQX treatment, which indicates thatPQX penetrates into the plasma membrane, followed by delocalization throughout the cells. MPQX was also able to penetrate the cell membrane, distribute throughout the cells and emit fluorescence, but did not show site-dependent intracellular fluorescence spectra. These results suggest thatPQX is a remarkable tool for investigating the local polarity environment in cells after appropriate conjugation to biomolecules.
Similar content being viewed by others
Notes and references
R. Y. Tsien, in Fluorescent and Photochemical Probes of Dynamic Biochemical Signals Inside Living Cells, ed. A. W. Czarnik, American Chemical Society, Washington, DC, 1993, pp. 130–146.
A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher, T. E. Rice, Chem. Rev., 1997, 97, 1515.
I. Johnson and M. T. Z. Spence, The Molecular Probes Handbook, Molecular Probes, Eugene, 11th edn, 2010.
G. Weber, Biochem. J., 1952, 51, 155.
J. M. Walker, Methods in Mol. Biol., 1997, 64, 189.
L.-J. Ma, Y. Li, L. Li, J. Sun, C. Tian, Y. Wu, Chem. Commun., 2008, 6345.
C.-H. Chui, Q. Wang, W.-C. Chow, M. C.-W. Yuen, K.-L. Wong, W.-M. Kwok, G. Y.-M. Cheng, R. S.-M. Wong, S.-W. Tong, K.-W. Chan, F.-Y. Lau, P. B.-S. Lai, K.-H. Lam, E. Fabbri, X.-M. Tao, R. Gambari, W.-Y. Wong, Chem. Commun., 2010, 46, 3538.
H. Khalfan, R. Abuknesha, M. Rand-Weaver, R. G. Price, D. Robinson, Histochem. J., 1986, 18, 497.
H. E. Katerinopoulos, Curr. Pharm. Des., 2004, 10, 3835.
K. E. Beatty, J. D. Fisk, B. P. Smart, Y. Y. Lu, J. Szychowski, M. J. Hangauer, J. M. Baskin, C. R. Bertozzi, D. A. Tirrell, ChemBioChem, 2010, 11, 2092.
S. Watanabe, S. Mizukami, Y. Hori, K. Kikuchi, Bioconjugate Chem., 2010, 21, 2320.
G. Signore, R. Nifosi, L. Albertazzi, B. Storti, R. Bizzarri, J. Am. Chem. Soc., 2010, 132, 1276.
A. Minta, J. P. Y. Kao, R. Y. Tsien, J. Biol. Chem., 1989, 264, 8171.
Y. Urano, M. Kamiya, K. Kanda, T. Ueno, K. Hirose, T. Nagano, J. Am. Chem. Soc., 2005, 127, 4888.
Y. Duan, M. Liu, W. Sun, M. Wang, S. Liu, Q. X. Li, Mini-Rev. Org. Chem., 2009, 6, 35.
T. Terai, T. Nagano, Curr. Opin. Chem. Biol., 2008, 12, 515.
H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim, J. Yoon, Chem. Soc. Rev., 2008, 37, 1465.
S. Watanabe, S. Mizukami, Y. Hori, K. Kikuchi, Bioconjugate Chem., 2010, 21, 2320.
W. Lin, X. Cao, Y. Ding, L. Yuan, L. Long, Chem. Commun., 2010, 46, 3529.
K. Kolmakov, V. N. Belov, J. Bierwagen, C. Ringemann, V. Müller, C. Eggeling, S. W. Hell, Chem.–Eur. J., 2010, 16, 158.
H. Sunahara, Y. Urano, H. Kojima, T. Nagano, J. Am. Chem. Soc., 2007, 129, 5597.
M. Kollmannsberger, K. Rurack, U. Resch-Genger, J. Daub, J. Phys. Chem. A, 1998, 102, 10211.
M. Kollmannsberger, K. Rurack, U. Resch-Genger, W. Rettig, J. Daub, Chem. Phys. Lett., 2000, 329, 363.
K. Rurack, M. Kollmannsberger, U. Resch-Genger, J. Daub, J. Am. Chem. Soc., 2000, 122, 968.
K. Rurack, M. Kollmannsberger, J. Daub, Angew. Chem., Int. Ed., 2001, 40, 385.
K. Kudo, A. Momotake, Y. Kanna, Y. Nishimura, T. Arai, Chem. Commun., 2011, 47, 3867.
G. Signore, R. Nifosì, L. Albertazzi, B. Storti, R. Bizzarri, J. Am. Chem. Soc., 2010, 132, 1276.
M. W. Berns, T. Krasieva, C.-H. Sun, A. Dvorniko, P. M. Rentzepis, J. Photochem. Photobiol., B, 2004, 75, 51.
Z. Diwu, Y. Lu, C. Zhang, D. H. Klaubert, R. P. Haugland, Photochem. Photobiol., 1997, 66, 424.
Z. Diwu, C.-S. Chen, C. Zhang, D. H. Klaubert, R. P. Haugland, Chem. Biol., 1999, 6, 411.
Q. Zhu, H.-S. Yoon, P. B. Parikh, Y.-T. Chang, S. Q. Yao, Tetrahedron Lett., 2002, 43, 5083.
R. A. Bozym, R. B. Thompson, A. K. Stoddard, C. A. Fierke, ACS Chem. Biol., 2006, 1, 103.
A. Hennig, H. Bakirci, W. M. Nau, Nat. Methods, 2007, 4, 629.
J. C. Tellis, C. A. Strulson, M. M. Myers, K. A. Kneas, Anal. Chem., 2011, 83, 928.
L. S. Hegedus, M. M. Greenberg, J. J. Wendling, J. P. Bullock, J. Org. Chem., 2003, 68, 4179.
L. Wang, W. C. Jackson, P. A. Steinbach, R. Y. Tsien, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 16745.
F. Kano, K. Takenaka, A. Yamamoto, K. Nagayama, E. Nishida, M. Murata, J. Cell Biol., 2000, 149, 357.
A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher, T. E. Rice, Chem. Rev., 1997, 97, 1515.
I. Johnson and M. T. Z. Spence, The Molecular Probes Handbook, Molecular Probes, Eugene, 11th edn, 2010.
W. J. Betz, F. Mao, C. B. Smith, Curr. Opin. Neurobiol., 1996, 6, 365.
S.-H. Son, Y. Yamagishi, M. Tani, M. Yuasa, K. Yamada, Chem. Lett., 2011, 40, 989.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kudo, K., Momotake, A., Tanaka, J.K. et al. Environmental polarity estimation in living cells by use of quinoxaline-based full-colored solvatochromic fluorophore PQX and its derivatives. Photochem Photobiol Sci 11, 674–678 (2012). https://doi.org/10.1039/c2pp05337c
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c2pp05337c