Abstract
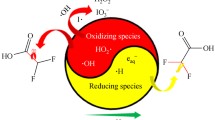
The perfluoroalkyl compounds (PFCs), perfluoroalkyl sulfonates (PFXS) and perfluoroalkyl carboxylates (PFXA) are environmentally persistent and recalcitrant towards most conventional water treatment technologies. Here, we complete an in depth examination of the UV-254 nm production of aquated electrons during iodide photolysis for the reductive defluorination of six aquated perfluoroalkyl compounds (PFCs) of various headgroup and perfluorocarbon tail length. Cyclic voltammograms (CV) show that a potential of +2.0 V (vs. NHE) is required to induce PFC oxidation and −1.0 V is required to induce PFC reduction indicating that PFC reduction is the thermodynamically preferred process. However, PFCs are observed to degrade faster during UV(254 nm)/persulfate (S2O82−) photolysis yielding sulfate radicals (E◦ = +2.4 V) as compared to UV(254 nm)/iodide (I-) photolysis yielding aquated electrons (E◦ = −2.9 V). Aquated electron scavenging by photoproduced triiodide (I3−), which achieved a steady-state concentration proportional to [PFOS]0, reduces the efficacy of the UV/iodide system towards PFC degradation. PFC photoreduction kinetics are observed to be dependent on PFC headgroup, perfluorocarbon chain length, initial PFC concentration, and iodide concentration. From 2 to 12, pH had no observable effect on PFC photoreduction kinetics, suggesting that the aquated electron was the predominant reductant with negligible contribution from the H-atom. A large number of gaseous fluorocarbon intermediates were semi-quantitatively identified and determined to account for ~25% of the initial PFOS carbon and fluorine. Reaction mechanisms that are consistent with kinetic observations are discussed.
Similar content being viewed by others
References
C. A. Moody and J. A. Field, Environ. Sci. Technol., 2000, 34, 3864–3870.
M. M. Schultz, D. F. Barofsky and J. A. Field, Environ. Eng. Sci., 2003, 20, 487–501.
N. Yamashita, S. Taniyasu, G. Petrick, S. Wei, T. Gamo, P. K. S. Lam and K. Kannan, Chemosphere, 2008, 70, 1247–1255.
C. J. Young, V. I. Furdui, J. Franklin, R. M. Koerner, D. C. G. Muir and S. A. Mabury, Environ. Sci. Technol., 2007, 41, 3455–3461.
V. I. Furdui, P. W. Crozier, E. J. Reiner and S. A. Mabury, Chemosphere, 2008, 73, S24–S30.
R. Guo, Q. F. Zhou, Y. Q. Cai and G. B. Jiang, Talanta, 2008, 75, 1394–1399.
M. H. Li, Environ. Toxicol., 2009, 24, 95–101.
V. Ochoa-Herrera, R. Sierra-Alvarez, A. Somogyi, N. E. Jacobsen, V. H. Wysocki and J. A. Field, Environ. Sci. Technol., 2008, 42, 3260–3264.
K. R. Rhoads, E. M. L. Janssen, R. G. Luthy and C. S. Criddle, Environ. Sci. Technol., 2008, 42, 2873–2878.
P. Rostkowski, N. Yamashita, I. M. K. So, S. Taniyasu, P. K. S. Lam, J. Falandysz, K. T. Lee, S. K. Kim, J. S. Khim, S. H. Im, J. L. Newsted, P. D. Jones, K. Kannan and J. P. Giesy, Environ. Toxicol. Chem., 2006, 25, 2374–2380.
M. K. So, S. Taniyasu, N. Yamashita, J. P. Giesy, J. Zheng, Z. Fang, S. H. Im and P. K. S. Lam, Environ. Sci. Technol., 2004, 38, 4056–4063.
C. D. Vecitis, H. Park, J. Cheng, B. T. Mader and M. R. Hoffmann, Front. Environ. Sci. Eng. China, 2009, 3, 129–151.
X. L. Zhao, J. D. Li, Y. L. Shi, Y. Q. Cai, S. F. Mou and G. B. Jiang, J. Chromatogr., A, 2007, 1154, 52–59.
V. Ochoa-Herrera and R. Sierra-Alvarez, Chemosphere, 2008, 72, 1588–1593.
H. Hori, E. Hayakawa, H. Einaga, S. Kutsuna, K. Koike, T. Ibusuki, H. Kiatagawa and R. Arakawa, Environ. Sci. Technol., 2004, 38, 6118–6124.
H. Hori, A. Yamamoto, E. Hayakawa, S. Taniyasu, N. Yamashita and S. Kutsuna, Environ. Sci. Technol., 2005, 39, 2383–2388.
H. Hori, A. Yamamoto, K. Koike, S. Kutsuna, I. Osaka and R. Arakawa, Water Res., 2007, 41, 2962–2968.
J. Chen and P. Zhang, Water Sci. Technol., 2006, 54, 317–325.
J. Chen, P. Zhang and L. Zhang, Chem. Lett., 2006, 35, 230–231.
R. Dillert, D. Bahnemann and H. Hidaka, Chemosphere, 2007, 67, 785–792.
H. Park, C. D. Vecitis, J. Cheng, W. Choi, B. T. Mader and M. R. Hoffmann, J. Phys. Chem. A, 2009, 113, 690–696.
Y. Qu, C. Zhang, F. Li, J. Chen and Q. Zhou, Water Res., 2010, 44, 2939–2947.
H. Hori, Y. Nagaoka, T. Sano and S. Kutsuna, Chemosphere, 2008, 70, 800–806.
T. Y. Campbell, C. D. Vecitis, B. T. Mader and M. R. Hoffmann, J. Phys. Chem. A, 2009, 113, 9834–9842.
J. Cheng, C. D. Vecitis, H. Park, B. T. Mader and M. R. Hoffmann, Environ. Sci. Technol., 2008, 42, 8057–8063.
J. Cheng, C. D. Vecitis, H. Park, B. T. Mader and M. R. Hoffmann, Environ. Sci. Technol., 2010, 44, 445–450.
H. Moriwaki, Y. Takagi, M. Tanaka, K. Tsuruho, K. Okitsu and Y. Maeda, Environ. Sci. Technol., 2005, 39, 3388–3392.
C. D. Vecitis, H. Park, J. Cheng, B. T. Mader and M. R. Hoffmann, J. Phys. Chem. A, 2008, 112, 4261–4270.
C. D. Vecitis, H. Park, J. Cheng, B. T. Mader and M. R. Hoffmann, J. Phys. Chem. C, 2008, 112, 16850–16857.
C. D. Vecitis, Y. Wang, J. Cheng, H. Park, B. T. Mader and M. R. Hoffmann, Environ. Sci. Technol., 2010, 44, 432–438.
L. C. T. Shoute, J. P. Mittal and P. Neta, J. Phys. Chem., 1996, 100, 3016–3019.
L. C. T. Shoute, J. P. Mittal and P. Neta, J. Phys. Chem., 1996, 100, 11355–11359.
P. L. Watson, T. H. Tulip and I. Williams, Organometallics, 1990, 9, 1999–2009.
P. Wardman, J. Phys. Chem. Ref. Data, 1989, 18, 1637–1755.
T. Yamamoto, Y. Noma, S. I. Sakai and Y. Shibata, Environ. Sci. Technol., 2007, 41, 5660–5665.
L. Lehr, M. T. Zanni, C. Frischkorn, R. Weinkauf and D. M. Neumark, Science, 1999, 284, 635–638.
J. W. T. Spinks and R. J. Woods, An Introduction to Radiation Chemistry, John Wiley & Sons, 3rd edn, 1990.
R. O. Rahn, M. I. Stephan, J. R. Bolton, E. Goren, P.-S. Shaw and K. R. Lykke, Photochem. Photobiol., 2003, 78, 146–152.
B. Guan, J. Zhi, X. Zhang, T. Murakami and A. Fujishima, Elec-trochem. Commun., 2007, 9, 2817–2821.
K. E. Carter and J. Farrell, Environ. Sci. Technol., 2008, 42, 6111–6115.
D. J. Barker, D. M. Brewis, R. H. Dahm and L. R. J. Hoy, Electrochim. Acta, 1978, 23, 1107–1110.
A. A. Pud, G. S. Shapoval, V. P. Kukhar, O. E. Mikulina and L. L. Gervits, Electrochim. Acta, 1995, 40, 1157–1164.
C. Combellas, F. Kanoufi and A. Thiebault, J. Phys. Chem. B, 2003, 107, 10894–10905.
M. C. Sauer, R. A. Crowell and I. A. Shkrob, J. Phys. Chem. A, 2004, 108, 5490–5502.
X. Y. Yu, Z. C. Bao and J. R. Barker, J. Phys. Chem. A, 2004, 108, 295–308.
R. O. Rahn, Photochem. Photobiol., 1997, 66, 450–455.
G. V. Buxton, C. L. Greenstock, W. P. Helman and A. B. Ross, J. Phys. Chem. Ref. Data, 1988, 17, 513–886.
J. Jortner, R. Levine, M. Ottolenghi and G. Stein, J. Phys. Chem., 1961, 65, 1232–1238.
T. Rigg and J. Weiss, J. Chem. Soc., 1952, 4198–4204.
H.-G. Boit, Beilstein Handbook of Organic Chemistry, Springer-Verlag, Berlin, 1975, Vol. 2.
Environment directorate joint meeting of the chemicals committee and the working party on chemicals pesticides and biotechnology, Organization for economic co-operation and development, Paris, 2002.
Material safety data sheet, Merck.
H. Li, D. Ellis and D. Mackay, J. Chem. Eng. Data, 2007, 52, 1580–1584.
Environmental and health assessment of perfluorooctane sulfonic acid and its salts, 3M report, 2003.
S. Kutsuna and H. Hori, Atmos. Environ., 2008, 42, 8883–8892.
K. U. Goss, Environ. Sci. Technol., 2008, 42, 5032–5032.
D. Brooke, A. Footitt and T. A. Nwaogu, Environmental risk evaluation report: perfluorooctane sulfonate (PFOS), Environment Agency, 2004.
P. Neta, R. E. Huie and A. B. Ross, J. Phys. Chem. Ref. Data, 1988, 17, 1027–1284.
E. Hayon, J. Phys. Chem., 1961, 65, 1937–1940.
E. Szajdzinska-Pietek and J. L. Gebicki, Res. Chem. Intermed., 2000, 26, 897–912.
L. Huang, W. B. Dong and H. Q. Hou, Chem. Phys. Lett., 2007, 436, 124–128.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available: Additional figures regarding the chemical actinometry, cyclic voltammetry, and GC-MS quantification of perfluorooctyl iodide. See DOI: 10.1039/c1pp05270e
Rights and permissions
About this article
Cite this article
Park, H., Vecitis, C.D., Cheng, J. et al. Reductive degradation of perfluoroalkyl compounds with aquated electrons generated from iodide photolysis at 254 nm. Photochem Photobiol Sci 10, 1945–1953 (2011). https://doi.org/10.1039/c1pp05270e
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c1pp05270e