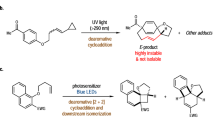
Abstract
A series of 4-aryl-1,1-dicyanobutenes (1a-1f) with different substituents were synthesized to control the intramolecular donor-acceptor or charge-transfer (C-T) interactions in the ground state. Photoexcitation of these C-T substrates led to competitive cyclization and rearrangement, the ratio being critically controlled by various environmental factors, such as solvent polarity, temperature and static pressure, and also by excitation wavelength and supramolecular confinement (polyethylene voids). In non-polar solvents, the rearrangement was dominant (>10: 1) for all examined substrates, while the cyclization was favoured in polar solvents, in particular at low temperatures. Selective excitation at the C-T band further enhanced the cyclization up to >50: 1 ratios. More importantly, the cyclization/rearrangement ratio was revealed to be a linear function of the C-T transition energy. However, the substrates with a sterically demanding or highly electron-donating substituent failed to give the cyclization product.
Similar content being viewed by others
Notes and references
N. J. Turro, V. Ramamurthy and J. C. Scaiano, Modern Molecular Photochemistry of Organic Molecules, University Science Books, Sausalito, California, 2010
P. Klan and J. Wirz, Photochemistry of Organic Compounds, John Willy and Sons, Ltd, 2009. For general reviews on photochemical organic transformations, see
T. Bach and J. P. Hehn, Photochemical reactions as key steps in natural product synthesis, Angew. Chem., Int. Ed., 2011, 50, 1000–1045
N. Hoffmann, Photochemical reactions as key steps in organic synthesis, Chem. Rev., 2008, 108, 1052–1103.
Y. Inoue, T. Wada, S. Asaoka, H. Sato and J. P. Pete, Photochirogenesis: Multidimensional control of asymmetric photochemistry, Chem. Commun., 2000, 251–259
H. Buschmann, H. D. Scharf, N. Hoffmann and P. Esser, The isoinversion principle a general model of chemical selectivity, Angew. Chem., Int. Ed. Engl., 1991, 30, 477–515
J. E. Leffler, The enthalpy-entropy relationship and its implications for organic chemistry, J. Org. Chem., 1955, 20, 1202–1231. Also see
Y. Inoue, T. Yokoyama, N. Yamasaki and A. Tai, An optical yield that increases with temperature in a photochemically induced enantiomeric Isomerization, Nature, 1989, 341, 225–226
Y. Inoue, H. Ikeda, M. Kaneda, T. Sumimura, S. R. L. Everitt and T. Wada, Entropy-controlled asymmetric photochemistry: Switching of product chirality by solvent, J.Am. Chem. Soc., 2000, 122, 406–407
Y. Inoue, E. Matsushima and T. Wada, Pressure and temperature control of product chirality in asymmetric photochemistry. Enantiodifferentiating photoisomer-ization of cyclooctene sensitized by chiral benzenepoly-carboxylates, J. Am. Chem. Soc., 1998, 120, 10687–10696.
N. J. Turro, V. Ramamurthy, W. Cherr and W. Farneth, The effect of wavelength on organic photoreactions in solution. Reactions from upper excited states, Chem. Rev., 1978, 78, 125–145. Also see
O. E. Alawode, C. Robinson and S. Rayat, Clean photodecomposition of 1_methyl-4-phenyl-1fl-tetrazole-5(4Ä)-thiones to carbodiimides proceeds via a biradical, J. Org. Chem., 2011, 76, 216–222
P. Wang, Y. Wang, H. Hu, C. Spencer, X. Liang and L. Pan, Sequential removal of photolabile protecting groups for carbonylswith controlled wavelength, J. Org. Chem., 2008, 73, 6152–6157.
A. Bauer, F. Westkamper, S. Grimme and T. Bach, Catalytic enantioselective reactions driven byphotoinduced electron transfer, Nature, 2005, 436, 1139–1140
A. G. Griesbeck and H. Heckroth, Stereoselective synthesis of 2-aminocyclobutanols via photocyclization of α-amido alkylaryl ketones: Mechanistic implications for the Norrish/Yang reaction, J. Am. Chem. Soc., 2002, 124, 396–403
J. N. Moorthy, S. Samanta, A. L. Koner, S. Saha and W. M. Nau, Intramolecular O-H ••• O hydrogen-bond-mediated reversal inthe partitioning of conformationally restricted triplet1, 4-biradicals and amplification of diastereodifferentiation in their lifetimes, J. Am. Chem. Soc., 2008, 130, 13608–13617
Y. Kawanami, T. C. S. Pace, J. Mizoguchi, T. Yanagi, M. Nishijima, T. Mori, T. Wada, C. Bohne and Y. Inoue, Supramolecular complexation and enantiodifferentiating photocyclodimerization of 2_anthracenecarboxylic acid with4-aminoprolinol derivatives as chiral hydrogen-bonding templates, J. Org. Chem., 2009, 74, 7908–7921.
P. Lakshminarasimhan, R. B. Sunoji, J. Chandrasekhar and V. Ramamurthy, Cation-π interaction controlled selective Geometric photoisomerization of diphenylcyclopropane, J. Am. Chem. Soc., 2000, 122, 4815–4816
S. Yamada, N. Uematsu and K. Yamashita, Role of Cation-pinteractions in the photodimerization of trans-4-styrylpyridines, J. Am. Chem. Soc., 2007, 129, 12100–12101
S. Yamada and Y. Tokugawa, Cation-π controlled solid-state photodimerization of 4-azachalcones, J. Am. Chem. Soc., 2009, 131, 20982099.
J. Mattay, Charge transfer and radical ions in photochemistry, Angew. Chem., Int. Ed. Engl., 1987, 26, 825–845
J. M. Masnovi, J. K. Kochi, E. F. Hilinski and P. M. Rentzepis, Reactive ion pairs from the charge-transfer excitation of electron donor-acceptor complexes, J. Am. Chem. Soc., 1986, 108, 1126–1135
M. Gonzalez-Bejar, S. E. Stiriba, M. A. Miranda and J. Perez-Prieto, Positive photocatalysis of a Diels-Alder reaction by quenching of excited naphthalene-indole charge-transfer complex with cyclohexadiene, Org. Lett., 2007, 9, 453456
N. Haga, H. Takayanagi and K. Tokumaru, Photoinduced electron transfer between acenaphthylene and1, 4-benzoquinones. Formation of dimers of acenaphthylene and 1: 1-adducts and effect of excitation mode on reactivity of thecharge-transfer complexes, J. Chem. Soc., Perkin Trans. 2, 2002, 734–745.
V. Ramamurthy and F. Eaton, Photochemistry and photophysics within cyclodextrin cavities, Acc. Chem. Res., 1988, 21, 300–306
J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, The cucurbit[n]uril family, Angew. Chem., Int. Ed., 2005, 44, 48444870
J. W. Lee, S. Samal, N. Selvapalam, H. J. Kim and K. Kim, Cucurbituril homologues and derivatives: New opportunitiesin supramolecular chemistry, Acc. Chem. Res., 2003, 36, 621–630
J. C. Scaianoand and H. Garcia, Intrazeolite photochemistry: Toward supramolecular controlof molecular photochemistry, Acc. Chem. Res., 1999, 32, 783–793
J. Sivagura, A. Natarajan, L. S. Kaanumalle, J. Shailaja, S. Uppili, A. Joyand and V. Ramamurthy, Asymmetric photoreactionswithin zeolites: Role of confinement and alkali metal ions, Acc. Chem. Res., 2003, 36, 509–521
R. G. Weiss, V. Ramamurthy and G. S. Hammond, Photochemistry in organic and confining media: A model, Acc. Chem. Res., 1993, 26, 530–536
V. Ramamurthy, R. G. Weiss and G. S. Hammond, A model for the influence of organized media on photochemical reactions, Adv. Photochem., 1993, 18, 67–236
C. H. Tung, L. Z. Wu, L. P. Zhangand B. Chen, Supramolecular system as microreactors: control of product selectivity in organic phototransformation, Acc. Chem. Res., 2003, 36, 39–47
L. R. MacGillivray, G. S. Papaefstathiou, T. Frisâc, T. D. Hamilton, D. K. Bucar, Q. Chu, D. B. Varshney and I. G. Georgiev, Supramolecular control of reactivity in the solid state: from templates to ladderanes to metal-organic frameworks, Acc. Chem. Res., 2008, 41, 280–291
M. Yoshizawa, J. K. Klosterman and M. Fujita, Functional molecular flasks: new properties and reactions within discrete, selfassembled hosts, Angew. Chem., Int. Ed., 2009, 48, 3418–3438
A. K. Sundaresan and V. Ramamurthy, Consequences of controlling free space within a reaction cavity with aremotealkyl group: Photochemistry of para-alkyl dibenzyl ketones within an organiccapsule in water, Photochem. Photobiol. Sci., 2008, 7, 1555–1564
N. J. Turro, Supramolecular organic and inorganic photochemistry: Radical pair recombination in micelles, electron transfer on starburst dendrimers, and the use of DNA as a molecular wire, Pure Appl. Chem., 1995, 67, 199–208.
H. Heitele, Dynamic solvent effects on electron-transfer reactions, Angew. Chem., Int. Ed. Engl., 1993, 32, 359–377
H. Sumi and R. A. Marcus, Dynamical effects in electron transfer reactions, J. Chem. Phys., 1986, 84, 4894–4914
R. A. Marcus, Chemical and electrochemical electron-transfer theory, Annu. Rev. Phys. Chem., 1964, 15, 155–196
R. A. Marcus, On the theory of oxidation-reduction reactions involving electron transfer. 1, J. Chem. Phys., 1956, 24, 966–978. See also
A. C. Benniston and A. Harriman, Charge on the move: How electron-transfer dynamics depend on molecular conformation, Chem. Soc. Rev., 2006, 35, 169–179.
S. V. Rosokha and J. K. Kochi, Fresh look at electron-transfer mechanisms viathe donor/acceptor bindings in the critical encounter complex, Acc. Chem. Res., 2008, 41, 641–653
J. K. Kochi, Innersphere electron transfer in organic chemistry. Relevance to electrophilic aromatic nitration, Acc. Chem. Res., 1992, 25, 39–47
J. K. Kochi, Charge-transfer excitation of molecular complexesin organic and organometallic chemistry, Pure Appl. Chem., 1991, 63, 255–264
M. Ottolenghi, Charge-transfer complexes in the excited state. Laser photolysis studies, Acc. Chem. Res., 1973, 6, 153–160.
H. Saito, T. Mori, T. Wada and Y. Inoue, Diastereoselective [2+2] photocycloaddition of stilbene to chiral fumarate. Direct versus charge-transfer excitation, J. Am. Chem. Soc., 2004, 126, 1900–1906
H. Saito, T. Mori, T. Wada and Y. Inoue, Switching of product’s chirality in diastereodifferentiating [2+2] photocycloaddition of (E)- versus(Z)-stilbene to chiral fumarate upon direct and chargetransfer-band excitation, Org. Lett., 2006, 8, 1909–1912.
H. Saito, T. Mori, T. Wada and Y. Inoue, Pressure control of diastereodifferentiating [2+2] photocycloaddition of (E)-stilbene to chiral fumarate upon direct and charge-transfer excitation, Chem. Commun., 2004, 1652–1653.
K. Matsumura, T. Mori and Y. Inoue, Wavelength control of diastereodifferentiating Paterno-Büchi reaction of chiral cyanobenzoates with diphenylethene through direct versus charge-transfer excitation, J. Am. Chem. Soc., 2009, 131, 17076–17077
K. Matsumura, T. Mori and Y. Inoue, Solvent and temperature effects on diastereodiffer-entiating PatermS-Biichi reaction of chiral alkyl cyanobenzoates with diphenylethene upon direct versus charge-transfer excitation, J. Org. Chem., 2010, 75, 5461–5469.
S. M. Hubig, R. Rathore and J. K. Kochi, Steric control of electron transfer. Changeover from outer-sphere to inner-sphere mechanisms in arene/quinone redox pairs, J. Am. Chem. Soc., 1999, 121, 617–626
R. Rathore, S. V. Lindeman and J. K. Kochi, Charge-transfer probes for molecular recognition via steric hindrance in donor-acceptor pairs, J. Am. Chem. Soc., 1997, 119, 9393–9404
S. Fukuzumi, C. L. Wong and J. K. Kochi, Unified view of Marcus electron transfer and Mulliken charge transfer theories in organometallic chemistry. Steric effects in alkylmetals as quantitative probes for outer-sphere and inner-sphere mechanisms, J. Am. Chem. Soc., 1980, 102, 2928–2939.
C. Bellucci, F. Gualtieri and A. Chiarini, Negative inotropic activityof para-substituted diethyl benzylphosphonates related to fostedil, Eur. J. Med. Chem., 1987, 22, 473–477.
European patent, EP 1787991 (A1), 2007.
G. Roman, J. G. Riley, J. Z. Vlahakis, R. T. Kinobe, J. F. Brien, K. Nakatsu and W. A. Szarek, Hemo oxygenase inhibition by 2-oxy-substituted 1-(1H-imidazol-1-yl)-4-phenylbutanes: Effect of halogen substitution in the phenyl ring, Bioorg. Med. Chem., 2007, 15, 3225*3234.
R. C. Cookson, D. E. Sadlar and K. Salisbury, The wavelength- and solvent-dependent photochemistry of 1, 1-dicyano-2-methyl-4-phenylbut-1-ene; reaction from two excited states, J. Chem. Soc., Perkin Trans. 2, 1981, 774–782.
C. Pourbaix, F. Carreaux and B. Carboni, Metal-catalyzed release of supported boronic acids for C-C bond formation, Org. Lett., 2001, 3, 803–805.
M. K. M. Dirania and J. Hill, Photo-acetalisation of α-aryloxyacetones, J. Chem. Soc., 1971, 1213–1215.
Y. Z. Chen and R. G. Weiss, Photoreactions of substituted o-cresyl acylates in cyclohexane and inpolyethylene films. The influences of intra- and inter-molecule ‘crowding’ effects, Photochem. Photobiol. Sci., 2009, 8, 916–925
C. A. Chesta, J. Mohanty, W. M. Nau, U. Bhattacharjee and R. G. Weiss, New insights into the mechanism of triplet radical-pair combinations. The persistent radical effect masks the distinction between in-cage and out-of-cage processes, J. Am. Chem. Soc., 2007, 129, 5012–5022
T. Mori, R. G. Weiss and Y. Inoue, Mediation of conformationallycontrolled photodecarboxylations of chiral and cyclic aryl esters bysubstrate structure, temperature, pressure, and medium constraints, J. Am. Chem. Soc., 2004, 126, 8961–8975.
For example, see: (a) J. W. Verhoeven, Through-bond charge transfer interaction and photoinduced charge separation, Pure Appl. Chem., 1986, 58, 1285–90
A. J. De Gee, J. W. Verhoeven, W. J. Sep and T. J. De Boer, Through-bond charge-transfer interaction in N-(p-methoxyphenylalkyl)pyridinium ions, J. Chem. Soc., Perkin Trans. 2, 1975, 579–583.
For an account, see: (a) S. Grimme, J. Antony, T. Schwabe and C. M. Lichtenfeld, Density functional theory with dispersion corrections for supramolecularstructures, aggregates, and complexes of (bio)organic molecules, Org. Biomol. Chem., 2007, 5, 741–758. Also see
S. Grimme, SemiempiricalGGA-type density functional constructedwith a long-range dispersion correction, J. Comput. Chem., 2006, 27, 1787–1799
S. Grimme, Accurate description of van der Waals complexesby density functional theory including empirical corrections, J. Comput. Chem., 2004, 25, 1463–1473.
D. Rehm and A. Weller, Kinetics of fluorescencequenching by electron and H-atom transfer, Isr. J. Chem., 1970, 8, 259–271
D. Rehm and A. Weller, Bonding and fluorescence spectra of hetereoexcimers, Z. Phys. Chem., 1970, 69, 183–200. See also
E. Prasad and K. R. Gopidas, Photoinduced electron transfer in hydrogen bonded donoracceptor systems. Study of the dependence of rate on free energy and simultaneous observation of the Marcus and Rehm-Weller behaviors, J. Am. Chem. Soc., 2000, 122, 3191–3196
S. M. Hubig and J. K. Kochi, Electron-transfer mechanisms with photoactivated quinones. The encounter complex versus the Rehm-Weller paradigm, J. Am. Chem. Soc., 1999, 121, 1688–1694.
P. Pasman, J. W. Verhoeven and T. J. de Boer, Fluorescence of intramolecular electron donor-acceptor systems; the importance of through-bond interaction, Chem. Phys. Lett., 1978, 59, 381385.
R. C. Cookson and J. E. Kemp, Retention of configuration at the migrating centre in both photochemical and thermal [1,3]-sigmatropic shift of a benzyl group. Relaxationof orbital symmetry control in an unsymmetrical ally1 system, Chem. Commun., 1971, 385–386.
C. Diedrich and S. Grimme, Systematic investigation of modern quantum chemical methods to predict electronic circular dichroism spectra, J. Phys. Chem. A, 2003, 107, 2524–2539
T. Mori, Y. Inoue and S. Grimme, Quantum chemical study on the circular dichroism spectra and specific rotation of donor-acceptor cyclophanes, J. Phys. Chem. A, 2007, 111, 7995–8006.
K. R. Brower, B. Gay and T. L. Konkol, The volume of activation in unimolecular decomposition reactions. Decarboxylation and demercuration, J. Am. Chem. Soc., 1966, 88, 1681–1685
K. R. Brower and J. S. Chen, The volume of Activation in Elimination Reactions, J. Am. Chem. Soc., 1965, 87, 3396–3401
K. R. Brower, The volume change of activation in the Claisen and Curtius rearrangements, J. Am. Chem. Soc., 1961, 83, 4370–4372
K. R. Browe, The volume change of activation in the decomposition of aromatic diazonium salts, J. Am. Chem. Soc., 1960, 82, 4535–4537.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of a themed issue in honour of Yoshihisa Inoue’s research accomplishments on the occasion of his 60th birthday.
Rights and permissions
About this article
Cite this article
Ito, T., Nishiuchi, E., Fukuhara, G. et al. Competitive photocyclization/rearrangement of 4-aryl-1,1-dicyanobutenes controlled by intramolecular charge-transfer interaction. Effect of medium polarity, temperature, pressure, excitation wavelength, and confinement. Photochem Photobiol Sci 10, 1405–1414 (2011). https://doi.org/10.1039/c1pp05038a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c1pp05038a