Abstract
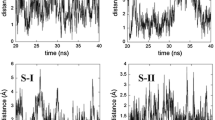
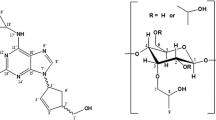
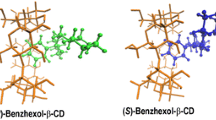
The chiral recognition ability of β-cyclodextrin (β-CyD) vs. S- and R-ketoprofen (KP) enantiomers has been studied by circular dichroism (CD), isothermal titration calorimetry (ITC) and NMR. The association constants of the 1 ∶ 1 complexes obtained from CD and ITC titration experiments resulted to be the same for both enantiomers within the experimental uncertainty. Well differentiated CD spectra were determined for the diastereomeric complexes. Their structure was assessed by molecular mechanics and molecular dynamics calculations combined with quantum mechanical calculation of the induced rotational strengths in the low energy KP:β-CyD associates, upon comparison of the calculated quantities with the corresponding experimental CD. The inclusion geometry is similar for both enantiomers with the aromatic carbonyl inserted in the CyD cavity, the monosubstituted ring close to the primary CyD rim and the carboxylate group exposed to the solvent close to the secondary rim. NMR spectra fully confirmed the geometry of the diastereomeric complexes. Tiny structural differences were sensibly probed by CD and confirmed by 2D ROESY spectra. Photoproduct studies with UV absorption and MS detection as well as nanosecond laser flash photolysis evidenced lack of chiral discrimination in the photodecarboxylation of KP within the cavity and formation of a photoaddition product to β-CyD by secondary photochemistry of 3-ethylbenzophenone.
Similar content being viewed by others
References
F. Bosca, M. L. Marin and M. A. Miranda, Photochem. Photobiol., 2001, 74, 637–655.
P. Ghezzi, G. Melillo, C. Meazza, S. Sacco, L. Pellegrini, C. Asti, S. Porzio, A. Marullo, V. Sabbatini, G. Caselli and R. Bertini, J. Pharmacol. Exp. Ther., 1998, 287, 969–974.
T. Suzuki, T. Okita, Y. Osanai and T. Ichimura, J. Phys. Chem. B, 2008, 112, 15212–15216.
C. D. Borsarelli, S. E. Braslavsky, S. Sortino, G. Marconi and S. Monti, Photochem. Photobiol., 2000, 72, 163–171.
S. Monti, S. Sortino, G. De Guidi and G. Marconi, New J. Chem., 1998, 22, 599–604.
S. Monti, S. Sortino, G. De Guidi and G. Marconi, J. Chem. Soc., Faraday Trans., 1997, 93, 2269–2275.
L. J. Martinez and J. C. Scaiano, J. Am. Chem. Soc., 1997, 119, 11066–11070.
S. Monti, I. Manet, F. Manoli, R. Morrone, G. Nicolosi and S. Sortino, Photochem. Photobiol., 2006, 82, 13–19.
S. Monti, F. Manoli, S. Sortino, R. Morrone and G. Nicolosi, Phys. Chem. Chem. Phys., 2005, 7, 4002–4008.
C. Festa, N. Levi-Minzi and M. Zandomeneghi, Gazz. Chim. Ital., 1996, 126, 599–603.
S. Monti, I. Manet, F. Manoli and S. Sortino, Photochem. Photobiol. Sci., 2007, 6, 462–470.
S. Monti, S. Ottani, F. Manoli, I. Manet, F. Scagnolari, B. Zambelli and G. Marconi, Phys. Chem. Chem. Phys., 2009, 11, 9104–9113.
S. Monti, I. Manet, F. Manoli, S. Ottani and G. Marconi, Photochem. Photobiol. Sci., 2009, 8, 805–813.
S. Monti, I. Manet, F. Manoli and G. Marconi, Phys. Chem. Chem. Phys., 2008, 10, 6597–6606.
S. Monti, I. Manet, F. Manoli, M. L. Capobianco and G. Marconi, J. Phys. Chem. B, 2008, 112, 5742–5754.
H. Huhnerfuss and M. R. Shah, J. Chromatogr., A, 2009, 1216, 481–502.
E. L. Izake, J. Pharm. Sci., 2007, 96, 1659–1676.
C. J. Easton and S. F. Lincoln, Chem. Soc. Rev., 1996, 25, 163–170.
A. M. Abushoffa, M. Fillet, P. Hubert and J. Crommen, J. Chromatogr., A, 2002, 948, 321–329.
E. Ameyibor and J. T. Stewart, J. Pharm. Biomed. Anal., 1998, 17, 83–88.
M. Blanco, J. Coello, H. Iturriaga, S. Maspoch and C. Perez-Maseda, J. Chromatogr., A, 1998, 799, 301–307.
M. Blanco, J. Coello, H. Iturriaga, S. Maspoch and C. Perez-Maseda, J. Chromatogr., A, 1998, 793, 165–175.
M. Blanco, J. Coello, H. Iturriaga, S. Maspoch and C. Perez-Maseda, Anal. Chim. Acta, 2000, 407, 233–245.
M. Blanco, J. M. Gonzalez, E. Torras and I. Valverde, Anal. Bioanal. Chem., 2003, 375, 157–163.
S. Rozou, S. Michaleas and E. Antoniadou-Vyza, J. Chromatogr., A, 2005, 1087, 86–94.
G. Marconi, S. Monti, F. Manoli, A. Degli Esposti and B. Mayer, Chem. Phys. Lett., 2004, 383, 566–571.
G. Marconi, S. Monti, F. Manoli, A. Degli Esposti and A. Guerrini, Helv. Chim. Acta, 2004, 87, 2368–2377.
P. Bortolus, G. Marconi, S. Monti and B. Mayer, J. Phys. Chem. A, 2002, 106, 1686–1694.
L. L. Costanzo, G. De Guidi, G. Condorelli, A. Cambria and M. Fama, Photochem. Photobiol., 1989, 50, 359–365.
Insight II, Accelrys software Inc., San Diego, CA, 2005.
W. Chen, C. E. Chang and M. K. Gilson, Biophys. J., 2004, 87, 3035–3049.
I. J. Tinoco, Adv. Chem. Phys., 1962, 4, 113–161.
Hyperchem 6.02, Hypercube Inc., Gainesville, FL, 2000.
J. H. Obbink and A. M. F. Hezemans, Chem. Phys. Lett., 1977, 50,133–137.
E. Skordi, I. D. Wilson, J. C. Lindon and J. K. Nicholson, Xenobiotica, 2004, 34, 1075–1089.
H. J. Schneider, F. Hacket, V. Rudiger and H. Ikeda, Chem. Rev., 1998, 98, 1755–1785.
80–90% of KP is complexed.
In ref. 5 the rate constant k2 had been assigned to the combination reaction of the radical pair made by the KP ketyl radical and the b-CyD radical, resulting from the H-abstraction from ß-CyD by the ketone triplet, to give a covalent KP-ß-CyD adduct with γmax = 205 nm.
S. Monti, N. Camaioni and P. Bortolus, Photochem. Photobiol., 1991, 54, 577–584.
S. Monti, L. Flamigni, A. Martelli and P. Bortolus, J. Phys. Chem., 1988, 92, 4447–4451.
G. Cosa, L. Llauger, J. C. Scaiano and M. A. Miranda, Org. Lett., 2002, 4, 3083–3085.
H. Suzuki, T. Suzuki, T. Ichimura, K. Ikesue and M. Sakai, J. Phys. Chem. B, 2007, 111, 3062–3068.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marconi, G., Mezzina, E., Manet, I. et al. Stereoselective interaction of ketoprofen enantiomers with β-cyclodextrin: ground state binding and photochemistry. Photochem Photobiol Sci 10, 48–59 (2011). https://doi.org/10.1039/c0pp00262c
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c0pp00262c