Abstract
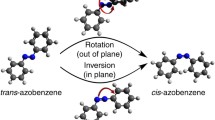
In a combined experimental and computational study of a group of para-substituted azobenzenes, the effects of substituents and solvent on the kinetics of thermal cis-to-transisomerisation have been examined and the success of DFT calculations in predicting kinetic parameters assessed. Mono-substituted species are predicted to isomerise by inversion in both non-polar and polar solvent, whereas for push-pull azobenzenes the mechanism is predicted to change from inversion to rotation on going from non-polar to polar solvent. Computed free energies of activation qualitatively reproduce experimental trends but do not quantitatively predict the kinetics of cis-trans isomerisation. The polarisable continuum model of solvation fails to predict the experimentally observed influence of solvent on the entropy of activation.
Similar content being viewed by others
References
G. S. Hartley, Cis form of azobenzene, Nature, 1937, 140, 281.
O. Sus, H. Steppan and R. Dietrich, Light induced reactions of diazo compounds, Justus Liebigs Ann. Chem., 1958, 617, 20–25.
E. R. Talaty and J. C. Fargo, Thermal Cis-trans isomerization of substituted azobenzenes: a correction of the literature, Chem. Commun., 1967, 65–66.
T. Asano, T. Okada, S. Shinkai, K. Shigematsu, Y. Kusano and O. Manabe, temperature and pressure dependences of the thermal cis-trans isomerization of azobenzenes which evidence an inversion mechanism, J. Am. Chem. Soc., 1981, 103, 5161.
D. Gegiou, A. Muszkat and J. Fischer, Temperature dependence of photoisomerization. v. the effect of substituents on the photoisomerization of stilbenes and azobenzenes, J. Am. Chem. Soc., 1968, 90, 3907.
N. Nishimura, T. Sueyoshi, H. Yamanaka, E. Imai, S. Yamamoto and S. Hasegawa, Thermal cis-to-trans isomerization of substituted azobenzenes. II Substituent and solvent effects, Bull. Chem. Soc. Jpn., 1976, 49, 1381–1387.
N. Nishimura, S. Kosako and Y. Sueishi, The thermal isomerization of azobenzenes. III Substituent, solvent, and pressure effects on the thermal isomerization of push-pull azobenzenes, Bull. Chem. Soc. Jpn., 1984, 57, 1617–1625.
P. D. Wildes, J. G. Pacifici, G. Irick and D. G. Whitten, Solvent and substituents effects on the thermal isomerization of substituted azobenzenes. A flash spectroscopy study, J. Am. Chem. Soc., 1971, 93, 2004–2008.
T. Sueyoshi, N. Nishimura, S. Yamamoto and S. Hasegawa, Further evidence of inversion mechanism for the cis-trans thermal isomerization of 4-dimethylaminoazobenzene derivatives. Additivity rule of substituent constants, Chem. Lett., 1974, 1131–1134.
N. Nishimura, S. Tanaka and Y. Sueishi, Evidence of an inversion mechanism for the thermal cis-trans isomerization of push-pull azobenzenes. A volumetric study, J. Chem. Soc., Chem. Commun., 1985, 903–904.
T. Asano and T. Okada, Further kinetic evidence for the competitive rotational and inversional z-e isomerization of substituted azobenzenes, J. Org. Chem., 1986, 51, 4454–4458.
K. S. Schanze, T. F. Mattox and D. G. Whitten, Solvent effects upon the thermal cis-trans isomerization and charge-transfer absorption of 4-(diethylamino)-4′-nitroazobenzene, J. Org. Chem., 1983, 48, 2808–2813.
Z. F. Liu, K. Morigaki, T. Enomoto, K. Hashimoto and A. Fujishima, Kinetic studies on the thermal cis-trans isomerization of an azo compound in the assembled monolayer film, J. Phys. Chem., 1992, 96, 1875–1880.
K. G. Yager and C. J. Barrett, Novel photo-switching using azobenzene functional materials, J. Photochem. Photobiol., A, 2006, 182, 250–261.
M. Irie, Photochromism: memories and switches-introduction, Chem. Rev., 2000, 100, 1683–1684.
M. B. Sponsler, in Optical Sensors and Switches, (Eds: V. Ramamurthy, K. S. Schanze), Marcel Dekker, New York and Basel, 2001, Chapter 8.
R. H. El Halabieh, O. Mermut and C. J. Barrett, Using light to control physical properties of polymers and surfaces with azobenzene chromophores, Pure Appl. Chem., 2004, 76, 1445–1465.
V. Balzani, A. Credi and M. Venturi, Light-powered molecular-scale machines, Pure Appl. Chem., 2003, 75, 541–547.
J. Dokic, M. Gothe, J. Wirth, M. V. Peters, J. Schwartz, S. Hecht and P. Saalfrank, Quantum chemical investigation of thermal cis-to-trans isomerization of azobenzene derivatives: substituent effects, solvent effects, and comparison to experimental data, J. Phys. Chem. A, 2009, 113, 6763–6773.
K. M. Tait, J. A. Parkinson, S. P. Bates, W. J. Ebenezer and A. C. Jones, The novel use of NMR spectroscopywith in situ laser irradiation to study azo photoisomerisation, J. Photochem. Photobiol., A, 2003, 154, 179–188.
K. M. Tait, J. A. Parkinson, D. I. Gibson, P. R. Richardson, W. J. Ebenezer, M. G. Hutchings and A. C. Jones, Structural characterisation of the photoisomers of reactive sulfonated azo dyes by NMR spectroscopy and DFT calculations, Photochem. Photobiol. Sci., 2007, 6, 1010–1018.
K. M. Tait, J. A. Parkinson, A. C. Jones, W. J. Ebenezer and S. P. Bates, Comparison of experimental and calculated 1H NMR chemical shifts of geometric photoisomers of azo dyes, Chem. Phys. Lett., 2003, 374, 372–380.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, GAUSSIAN 03 (Revision B.02), Gaussian, Inc., Wallingford, CT, 2004.
A. D. Becke, Density-functional exchange-energy approximation with correct asymptotic behaviour, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098.
C. Lee, W. Yang and R. G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B: Condens. Matter, 1988, 37, 785.
S. Miertus, E. Scrocco and J. Tomasi, Electrostatic interaction of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects, Chem. Phys., 1981, 55, 117–129.
M. Cossi, G. Scalmani, N. Rega and V. Barone, New developments in the polarizable continuum model for quantum mechanical and classical calculations on molecules in solution, J. Chem. Phys., 2002, 117, 43–54.
M. Poprawa-Smoluch, J. Baggerman, H. Zhang, H. P. A. Maas, L. DeCola and A. M. Brouwer, Photoisomerization of disperse red 1 studied with transient absorption spectroscopy and quantum chemical calculations, J. Phys. Chem. A, 2006, 110, 11926–11937.
K. Matczyszyn, W. Bartkowiak and J. Leszczynki, Influence of the environment on kinetics and electronic structure of asymmetric azobenzene derivatives - experiment and quantum-chemical calculations, J. Mol. Struct., 2001, 565–566, 53–57.
C. Reichardt, Empirical parameters of the polarity of solvents, Angew. Chem., Int. Ed. Engl., 1965, 4, 29–40.
R. G. Pearson, Influence of the solvent on rates of ionization, entropies, and heats of activation, J. Chem. Phys., 1952, 20, 1478–1480.
Author information
Authors and Affiliations
Additional information
This article is published as part of a themed issue in appreciation of the many important contributions made to the field of molecular photophysics by Jan Verhoeven.
Electronic supplementary information (ESI) available: NMR spectra. See DOI: 10.1039/c0pp00056f
Rights and permissions
About this article
Cite this article
Wazzan, N.A., Richardson, P.R. & Jones, A.C. Cis-Trans isomerisation of azobenzenes studied by laser-coupled NMR spectroscopy and DFT calculations. Photochem Photobiol Sci 9, 968–974 (2010). https://doi.org/10.1039/c0pp00056f
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c0pp00056f